Gastroenterology and Hepatology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2836-2888 | Journal DOI: 10.61148/2836-2888/GHR
Andres Valencia Uribe 1 *, Lazaro Antonio Arango Molano 2
1Gastroenterology fellow universidad de caldas. General surgery specialist. Manizales, colombia.
2Gastroenterologist. General surgeon. Chief gastroenterology specialization universidad de caldas. Chief advanced endoscopy unión de cirujanos, oncólogos de occidente. President asociación colombiana de endoscopia digestiva. Manizales, colombia.
*Corresponding author: Andres Valencia Uribe, Gastroenterology fellow universidad de caldas. General surgery specialist. Manizales, colombia.
Received date: December 17, 2021
Accepted date: December 30, 2021
published date: January 20, 2022
Citation: Andres V Uribe and Arango Molano.L.A. (2022) “Proposal of A Stomach Cancer Screening Program for A Latin American Population”, J of Gastroenterology and Hepatology Research, 3(2); DOI: http;//doi.org/01.2022/2.10130
Copyright: © 2022 Andres Valencia Uribe. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Gastric cancer is an importantly prevalent and incident disease with a high mortality rate, it is the fourth cause of cancer, and the second cause of death from malignant tumors in the world. Many risk factors are associated with gastric adenocarcinoma and its pathogenesis is multifactorial. Diagnosis of gastric cancer is frequently made in advanced stages of the disease, implying a poor survival rate. Early detection is of great significance and utility in reducing mortality, but there are no global screening strategies. Screening and surveillance for premalignant lesions is of special interest because of their potential progression to gastric cancer and the possibility to avoid it. Gastric cancer screening strategies are useful in places with a high incidence. Therefore, it is necessary to adopt early detection programs for gastric cancer applied to Latin America. So, we propose a program with that purpose with a comprehensive vision as a route of admission, diagnosis, management, follow-up, and evaluation of results and impact, in favor of the health status of patients at potential risk of gastric cancer.
1. Introduction:
Gastric cancer is a disease with a high mortality rate, it is the fourth cause of cancer, and the second cause of death from malignant tumors in the world. Although many risk factors are associated with gastric adenocarcinoma, there are still no definite causes. The pathogenesis is most likely multifactorial. The existence of a sequence of histological premalignant changes has been widely postulated, progressing from atrophic gastritis to intestinal metaplasia and finally to gastric adenocarcinoma, these premalignant histological changes may be necessary, but they are not clearly sufficient for the development of this entity [1,3,5]. Gastric cancer results from successive changes that the gastric mucosa undergoes since childhood, whose slow and progressive progression originates deep transformations and ultimately originates the tumor [1,2,3,4]. Helicobacter pylori is strongly implicated in the etiology of gastric cancer [23,25]. The risk of infection throughout life in developed countries is 40 to 60%, but it is very high in developing countries where it can reach up to 90%, as we can see in Latin America [9, 17, 25, 26].
The diagnosis of gastric cancer in advanced stages of the disease implies a worse survival rate. Early detection is of great significance and utility in reducing mortality, but there are no global screening strategies [1,5,6,7,8].
Identification of premalignant lesions is important for the purpose of screening and surveillance. The annual progression of them to gastric cancer is estimated between 0 and 1.8% for atrophic gastritis, 0 to 10% for intestinal metaplasia and 0 to 73% for dysplasia; with an annual incidence of 0.2% for atrophic gastritis, 0.25% for intestinal metaplasia, 0.6% for mild to moderate dysplasia, and 6% for severe dysplasia. Endoscopic surveillance in these cases ensure early detection and curable stages of the disease [9, 10, 11, 12, 13].
Gastric cancer screening strategies have proven to be useful in regions where there is a high incidence [13, 14, 16, 18, 19, 20, 21, 27]. Therefore, it is necessary to adopt an early detection program for gastric cancer applied to our environment.
The program presented below brings together a comprehensive vision as a route of admission, diagnosis, management, follow-up, and results in favor of the health status of patients at potential risk of developing or with gastric cancer.
2. Objectives:
2.1. General:
Implement a care route that allows early detection of gastric cancer in a developing country population, seeking to reduce the complications that may occur in patients through the identification of risks, timely intervention, follow-up, accompaniment and education for the patient and his family.
2.2. Specific:
● Facilitate the follow-up of patients with risk factors for stomach cancer.
● Early identification of patients with a suspected diagnosis of stomach cancer.
● Provide systematic and organized management to reduce access barriers for patients with gastric cancer diagnosis.
● Carry out a comprehensive and protocolized management of the patient with a suspected or established diagnosis of stomach cancer, to improve survival, reduce morbidity and mortality, decrease the number of hospital admissions and improve their quality of life.
● Provide psychosocial support for patients, their families and caregivers according to the different stages of disease, facilitating feedback with specific and advanced evidence - based treatment options.
3. Justification:
Contemporarily, the initiatives for the management, control and monitoring of patients should be framed within the model determined by the triple health goal:
In accordance with the first component, individualized care focused on quality, safety, equity, opportunity, generated by interdisciplinary professionals suitable in the provision of health services guarantees the differential framework in the care experience.
Regarding the improvement of population health, the program clearly justifies what to do in the route promulgated for early detection, care, monitoring and quantification of the impact of diagnostic and therapeutic interventions, which guarantee to maintain their vital situation in accordance with quality of life and adequate health standards.
In an integrative way, taking the patient through an adequate control path, necessarily implies the reduction of the costs derived from the care process. This aspect takes visibility with the aim of the program to improve the successful detection of factors of risk, premalignant lesions and early gastric cancer to improve prognosis and reduce morbidity and mortality related s with advanced gastric cancer, and optimize patterns treatment and close monitoring [21, 22, 23, 24, 25, 27].
Gastric cancer early detection programs have shown great utility and impact on the prognosis of gastric cancer, an example given by the Japanese program, in which approximately 1 case is detected for every 800 patients examined, half of them in the early stage (the latter explains the positive impact on mortality) . In the Western world, the overall 5-year survival after the diagnosis of gastric cancer is less than 25%, with detection programs Japan has achieved that survival in advanced gastric cancer reaches around 52%, and when an effective early detection survival reaches 95% [2, 6, 7, 8, 12, 13].
Endoscopic evaluation of the esophagus, stomach and duodenum with biopsy is the standard method for the diagnosis of cancer gastric. The technique is highly sensitive when performed by experienced specialists, and allows detection of early stages lesions [14, 15, 16, 17, 20, 21, 25, 27].
It is necessary to establish the importance of control with follow-up by an interdisciplinary group that guides the patient and his family in the identification of alarm signs, recognition of the importance of not abandoning the established treatment and follow-up , under a care scheme focused on individualization of the patient, their environment, as well as the need of health personnel to keep the patient in the best conditions of well - being and management of their pathology.
4. SCOPE:
From patient recruitment (induced demand, referral for general medicine, specialties, subspecialties, hospitalization), to referral to the specialty of oncology or discharge of the patient after follow-up.
5. Target Population:
Population older than 18 years old with uninvestigated dyspepsia and no response to optimal initial management with a proton pump inhibitor. Population over 35 years of age with dyspeptic symptoms without previous studies, regardless of the response to medical management. People with risk factors for gastric cancer, with warning signs (abnormal weight loss, persistent emesis), suspected gastric preneoplastic lesions, and / or diagnosis of gastric cancer.
6. Interdisciplinary Team:
● General Practitioner.
● Gastroenterologist.
● Chief nurse.
● Assistant Nurse.
● Oncologist.
● Oncologic surgeon.
6.1. Functions of The Professionals of The Interdisciplinary Team:
General practitioner (primary health care):
Gastroenterologist:
Chief nurse:
Assistant nurse:
Oncologist:
Oncological Surgeon:
7. Inclusion and Exclusion Criteria:
Inclusion:
■ Lynch syndrome (hereditary nonpolyposis colorectal cancer).
■ Familial adenomatous polyposis (FAP).
■ Diffuse hereditary gastric cancer.
■ Hereditary breast and ovarian cancers.
■ BRCA1 and BRCA2 mutations.
■ Li-Fraumeni syndrome.
■ Peutz-Jeghers syndrome.
○ Rich salty foods cured meats (nitrates and nitrites), smoked foods.
○ Antecedent person l of pernicious anemia or achlorhydria.
○ Smoking.
○ Alcoholism.
○ Obesity (mainly in men).
○ Atrophic chronic gastritis (H. pylori). Intestinal metaplasia - Mucous dysplasia.
○ MALT-type gastric lymphoma.
○ Previous gastric surgery, especially for acid-peptic disease.
○ Ménétrier's disease (hypertrophic gastropathy).
○ Blood type.
○ Epstein Barr virus.
○ Gastric adenomatous polyps.
○ Industries workers (metal, carbon, rubber, silica, lead, asbestos).
○ Common variable immunodeficiency.
4. Voluntary acceptance to enter the program.
Exclusion:
8. Attention Route:
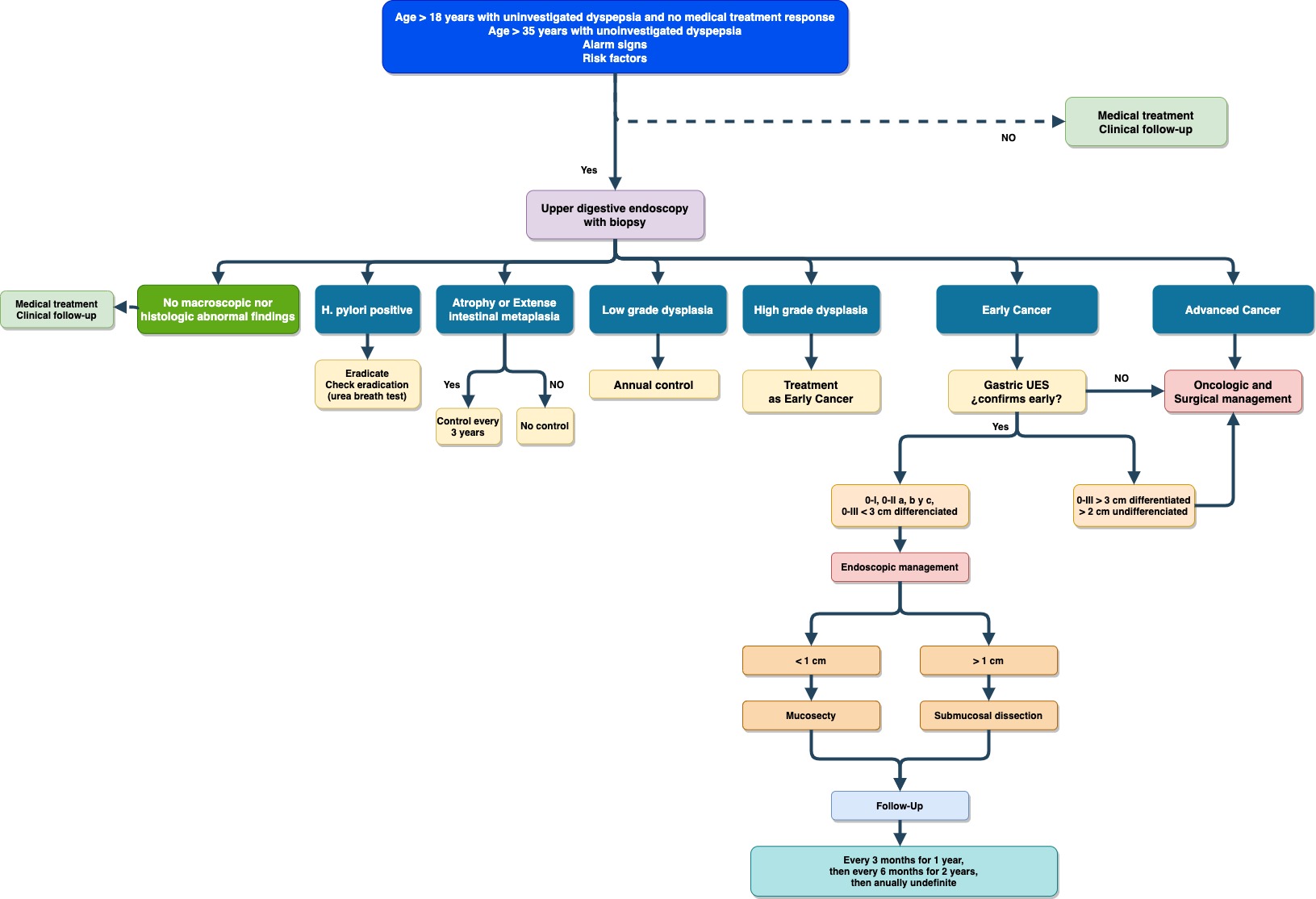
9. Process:
Step by step patient care, from entry until their release for the program of detection of gastric cancer, comprising the following steps:
9.1. Admission to the program:
9.1.1. Patient uptake:
Responsible: General practitioner (outpatient consultation, emergencies, hospitalization). Specialist physician (family medicine, internal medicine, general surgery, gastroenterology).
● Complete clinical evaluation of the patient and verification of inclusion criteria as potential members of the gastric cancer detection program.
● Feed the database of patients referred or indicated for admission to the stomach cancer detection program.
● Follow-up patients directed (outpatient, emergency and hospitalization ).
● Assign an assessment appointment with the nurse to verify criteria and complete registration in the program.
9.1.2. Assessment of inclusion and exclusion criteria:
Responsible:
Chief Nurse:
● Evaluate and verify inclusion and exclusion criteria of the patient.
● Comprehensively assess patients.
● Provide program information to the patient and how it works.
● Assign assessment appointment for the first time or for tracking control as required.
● Evaluate previously generated orders and / or pending studies to manage them.
9.1.3. Medical consultation for the first time:
Responsible:
Medical leader of the program (General Practitioner or Gastroenterologist):
● Carry out an assessment of symptoms, presence of risk factors and warning signs for gastric cancer.
● Evaluate response to previous treatments and their quality.
● Determine indication and relevance of performing an upper digestive endoscopic study.
● Evaluation of previous studies, including endoscopies with their respective histopathology reports.
● Determine the need and timing of specialized evaluation.
● Explain the patient and their caregiver: Current diagnosis and plan to follow.
● Define patient follow-up according to the medical evaluation performed.
9.2. Follow-up:
Directed follow-up in accordance with identified risks, and endoscopic and histopathologic findings, defining frequency of consultations, specialized evaluation, additional studies, etc.
9.2.1. Follow-up by General Medicine (Medical Leader):
9.2.2. Follow-up by Gastroenterology:
9.2.3. Monitoring by nursery:
9.3. Discharge:
10. Indicators and Measurement of The Impact of The Program:
The indicators to be evaluated in the measurement of impact of the program will be: