Gastroenterology and Hepatology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2836-2888 | Journal DOI: 10.61148/2836-2888/GHR
Lázaro Antonio Arango Molano MD, FASGE1, Ángel Rojas Espinosa, MD2*
1General Surgeon, Clinical-Surgical Gastroenterologist, Universidad de Caldas, Manizales, Colombia
2General Surgeon, Clinical-Surgical Gastroenterology Fellow, Universidad de Caldas, Manizales, Colombia.
*Corresponding author: Ángel Rojas Espinosa, General Surgeon, Clinical-Surgical Gastroenterology Fellow, Universidad de Caldas, Manizales, Colombia.
Received date: December 17, 2021
Accepted date: December 28, 2021
published date: January 18, 2022
Citation: Arango Molano. L.A and Ángel R Espinosa. (2022) “Screening Strategies and Integral Health Care for Colorectal Cancer”, J of Gastroenterology and Hepatology Research, 3(1); DOI: http;//doi.org/12.2022/2.10129
Copyright: © 2022 Ángel Rojas Espinosa. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
The incidence of colorectal cancer is highly variable worldwide and is closely related to the Western lifestyle due to its low hereditary component.
It is estimated that more than 1.5 million patients are diagnosed with colorectal cancer and more than 500,000 die from this disease worldwide.
The mainstays of treatment are early diagnosis with endoscopic resection, surgery or neoadjuvant or adjuvant therapies.
This review is aimed at primary care physicians and mainly at early detection programs, with which it is hoped to disseminate and standardize actions aimed at cancer control.
Introduction:
Colorectal cancer (CRC) incidence and mortality vary considerably depending on the world population studied. Worldwide, the highest incidence of CRC is described in Australia, New Zealand, North America and the lowest in Africa and Central and South Asia [1]
Approximately 52980 Americans are expected to die from CRC annually, although due to timely endoscopic detection and management, the current rate has decreased since 1990 [2] But it remains the third most common cause of cancer death in the United States.
The literature suggests guidelines to begin CRC screening in those over 45 years of age, since the 50 to 60 years interval is the peak of CRC incidence. [3] The CRC is diagnosed after the onset of symptoms or in a screening program, which can be performed by colonoscopy or laboratory.
In Colombia during 2019 (Fondo Colombiano de Enfermedades de Alto Costo) registered 279,155 people with cancer in Colombia, of which 265,863 were cases of invasive cancer. [4]
The median age of new cancer cases was 61 years. Of the cases (n=17,487) 59.90% were diagnosed in women, with a male: female ratio of 0.6:1 [4-5]
In the case of CRC, it ranked third in the period analyzed, including both in situ and invasive cases, 19,147 prevalent cases were reported, of which approximately 12% (n=2,285) were new cases. In terms of mortality, 1,901 deaths were reported. [4]
The 34.79% were diagnosed in stage III, with the median age being similar in all stages 62 - 64 years. [4]
Risk Factors:
There are individual risk factors, such as genetic load and environmental exposure, which together can develop or increase the risk of CRC onset. It is well known that most CRC is sporadic rather than familial.
Early onset CRC includes a history of first-degree relatives, hyperlipidemia, obesity, alcohol consumption, consumption of processed red meats. [8]
Modifiable Risk Factors:
Obesity: It is indicated that weight gain in early and middle adulthood is associated with an increased risk of CRC.
Red and processed meat: The World Health Organization classified the consumption of processed meat as carcinogenic and the consumption of red meat as probable carcinogenic, indicating that for every 50 grams of processed meat per day and 100 grams of red meat consumed per day, the risk of CRC is increased by 16% and 12%, respectively.
Tobacco: It has a higher incidence and mortality for CRC, with a higher predominance in mortality for rectal cancer than for colon cancer.
Alcohol: The risk for CRC is in moderate drinkers (two or three drinks a day) or heavy drinkers (more than four drinks a day) or in general more than 10 gr/day of alcohol. [8-9]
Non-Modifiable Risk Factors:
Hereditary CRC syndromes: Most of them are inherited autosomal dominant and are associated with high risk of CRC (Familial adenomatous polyposis, Lynch syndrome, MUTYH-associated polyposis, Hereditary breast and ovarian cancer syndrome) [10].
Familial Adenomatous Polyposis: These are 1% of CRC, with CRC occurring in 90% before the age of 45 years and is caused by a germline mutation in the adenomatous polyposis coli (APC) gene. [11]
MUTYH-associated polyposis: Associated with a mutation of the base excision repair gene (MUTYH) of autosomal recessive inheritance, typically more than 15 and less than 100 adenomas are present, with late onset between 40 - 50 years with no descriptions of early cases. [12]
Lych syndrome: It is more frequent than familial adenomatous polyposis, and represents 3% of colon adenocarcinomas, also called hereditary non-polyposis CRC, it is due to pathogenic variants of some genes involved in the repair of errors in DNA replication. [13]
Ulcerative Colitis: Pancolitis confers a five to fifteen-fold increased risk of CRC compared to the average risk, and the incidence begins to increase as the duration of disease exceeds 10 to 20 years. [14]
Abdominopelvic Radiation: Survivors of cancer received in childhood or adulthood have an increased risk of gastrointestinal neoplasms, most of which are CRC, it is suggested that follow-up in these patients should be at five years after completion of radiation therapy or at 30 years of age. [8]
Colorectal Cancer Screening:
CRC cancer is a preventable disease in those over 45 years of age, based on screening plans, the main risk factor which is unmodifiable is age, in those over 50 years of age. [15]
When detected in early stages, the overall survival rate is 90%. There are fecal tests such as the fecal immunochemical test (FIT) with a sensitivity of 81% compared to the sensitivity of guaiac fecal occult blood in stool on three occasions (gFOBT), which reaches 61%. [15-16]
The sensitivity for detecting colorectal adenomas was 96% for FIT and 97% for gFOBT [15]
Currently, stool DNA testing is a relatively new method for detecting mutations in the adenomatous polyposis coli (APC) gene.
The guaiac fecal occult blood test (gFOBT):
Three consecutive stool samples should be taken annually, but patients should be instructed to avoid taking non-steroidal anti-inflammatory drugs (NSAIDs), red meat consumption during the three days prior to the test, and vitamin C intake should also be avoided as this can lead to false positives. [17]
Fecal Immunochemical Test (FIT):
This functional test uses antibodies against hemoglobin to detect occult blood in feces, so it does not require dietary restrictions since it only detects human blood and at the colorectal level, so it has fewer false positive results in endemic populations with Helicobacter pylori. [18]
Fecal DNA Test:
This test is multipurpose, since it not only detects human blood in stool, but also certain genetic mutations in the DNA of large adenoma cells and colorectal cancer cells.
|
|
Target Population |
Sensitivity / Specificity |
Cost |
|
gFOBT |
Average Risk |
30-70% / 97 % |
5- 23 US |
|
FIT |
Average Risk / High Risk |
76% / 95% |
22 – 24 US |
|
Fecal DNA Test
|
Average Risk |
55 - 88% / 94 % |
150 US |
Patel SS, et al. Cost Effectiveness of Colorectal Cancer Screening Strategies. Cancer Control. 2015;22(2):248-258 Tomado y modificado de: Patel SS, et al. Cost Effectiveness of Colorectal Cancer Screening Strategies. Cancer Control. 2015;22(2):248-258
Image Tests:
Colonoscopy:
It is an efficient test when it is directed to the appropriate population and meets the quality criteria being the cannulation of the cecum and withdrawal minimum 7 - 9 minutes.
The error rate in colorectal cancer detection is between 0.2 - 5%. [19]
Flexible Sigmoidoscopy:
It has been shown to reduce mortality in colorectal cancer by 18 - 23% since it can also observe the mucosa and remove the polyps visible there, in combination with stool tests. [19-20]
It usually has very low complications and may not require sedation for its performance.
Tomography (CT) Colonography:
It is a minimally invasive option, requires a CT scan and 3D reconstruction, is less invasive than endoscopic procedures and does not require recovery time.
It can detect polyps larger than 10 mm and other abnormal findings should be carried colonoscopy studies. [21]
|
|
Target Population |
Sensitivity / Specificity |
Cost |
|
Colonoscopy |
High Risk |
95% / 100 % |
161 – 1218 US |
|
Flexible Sigmoidoscopy
|
Average Risk |
60 – 95 % / 90% |
161 – 224 US |
|
CT Colonography
|
Average Risk |
80 – 95 % / 90% |
540 – 900 US |
Patel SS, et al. Cost Effectiveness of Colorectal Cancer Screening Strategies. Cancer Control. 2015;22(2):248-258 Tomado y modificado de: Patel SS, et al. Cost Effectiveness of Colorectal Cancer Screening Strategies. Cancer Control. 2015;22(2):248-25
1. Colorectal Cancer Screening Algorithm:
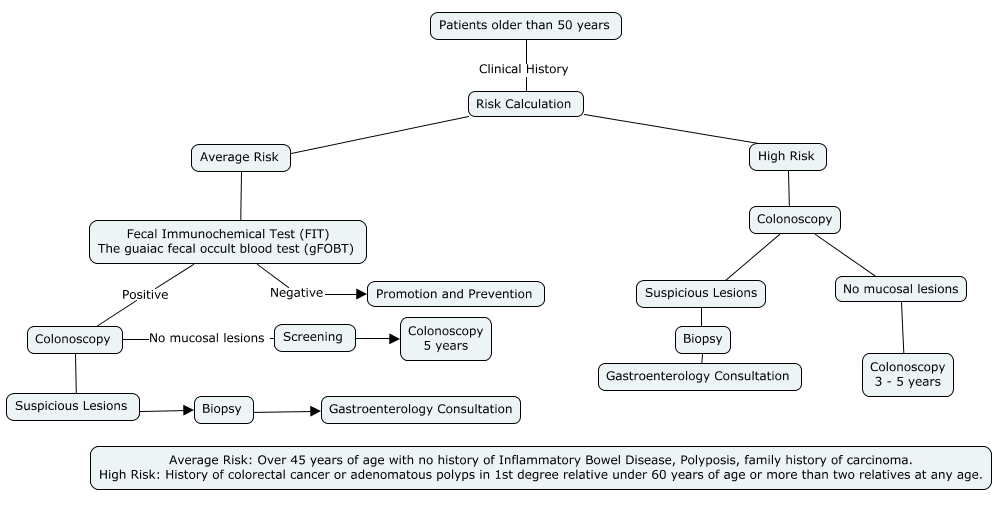
* Article Authors
Colorectal Cancer:
Early Stage:
Resection of colon or rectal polyps in long-term repeat surveillance programs may lead to detection of malignant polyps or advanced asymptomatic high-risk asymptomatic adenomas. Localized disease is limited to the submucosal or muscularis propria (Stage 1). [22]
Early stage is defined as completely resected disease which has no underlying organ or lymph node involvement, staging criteria for colorectal cancer are established by the American Joint Committee on Cancer (AJCC).
|
Definition |
|
|
T Stage |
|
|
Tx |
No information about local tumour infiltration available |
|
Tis |
Tumour restricted to mucosa, no infiltration of lamina muscularis mucosae |
|
T1 |
Infiltration through lamina muscularis mucosae into submucosa, no infiltration of lamina muscularis propria |
|
T2 |
Infiltration into, but not beyond, lamina muscularis propria |
|
T3 |
Infiltration into subserosa or non-peritonealised pericolic or perirectal tissue, or both; no infiltration of serosa or neighbouring organs |
|
T4a |
Infiltration of the serosa |
|
T4b |
Infiltration of neighbouring tissues or organs |
|
N Stage |
|
|
Nx |
No information about lymph node involvement available |
|
N0 |
No lymph node involvement |
|
N1a |
Cancer cells detectable in 1 regional lymph node |
|
N1b |
Cancer cells detectable in 2–3 regional lymph nodes |
|
N1c |
Tumour satellites in subserosa or pericolicor perirectal fat tissue, regional lymph nodes not involved |
|
N2a |
Cancer cells detectable in 4–6 regional lymph nodes |
|
N2b |
Cancer cells detectable in 7 or greater regional lymph nodes |
|
M Stage |
|
|
Mx |
No information about distant metastases available |
|
M0 |
No distant metastases detectable |
|
M1a |
Metastasis to 1 distant organ or distant lymph nodes |
|
M1b |
Metastasis to more than 1 distant organ or set of distant lymph nodes or peritoneal metastasis |
|
Classification of colorectal cancers according to local invasion depth (T stage), lymph node involvement (N stage), and presence of distant metastases (M stage) |
|
Table 3: Sobin LH, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours, 7th edn. New York: Wiley-Blackwell, 2009.
Rectal endoscopic ultrasound:
comprehensively evaluates the rectal wall layers and T staging and defines the treatment of tumors, tumors located at T1 are suitable for endoscopic surgery [24].
2. Non-metastatic Colon Cancer Follow-Up Algorithm:
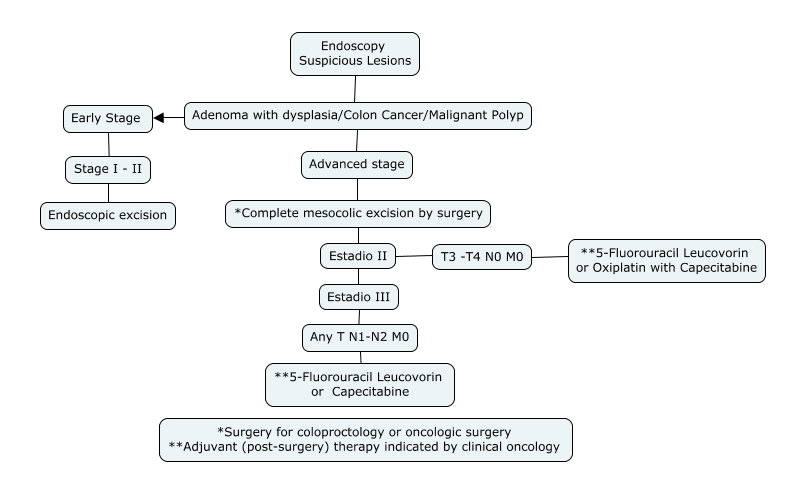
* Article Authors
Surgical management of primary colon cancers without systemic involvement is mostly treated by surgical management with complete intestinal and mesocolic resection together with ligation of arteries and veins as close as possible to the vascular trunk, thus improving survival. [24-25]
It is known that surgical resection allows an excellent oncologic outcome with a 5-year cancer-specific survival rate of 91.4% in stage II and 70.2% in stage III. [26]
3. Non-metastatic Rectal Cancer Follow-up Algorithm:
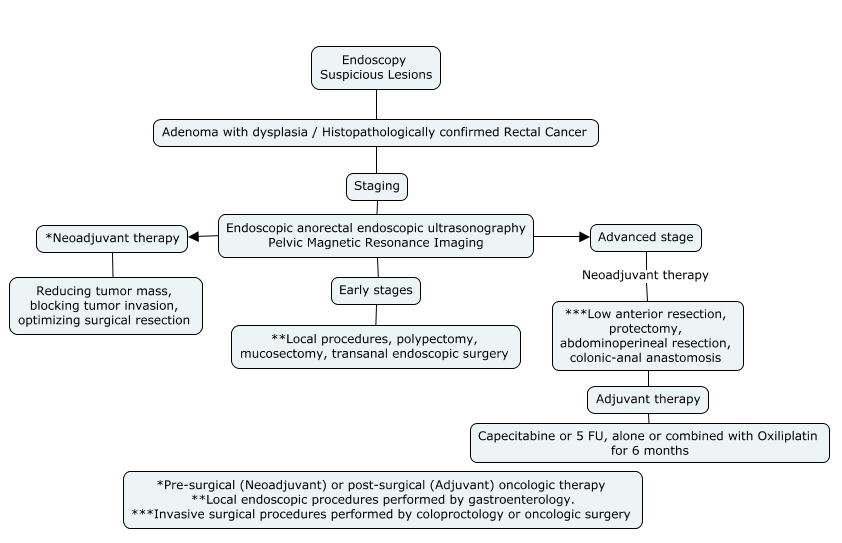
* Article Authors
Rectal cancers can be divided into four groups: very early (some T1), early (T1-2), intermediate (most T3) and locally advanced (most T4), but important factors other than T stage, such as distance from the anal verge, circumferential margin, nodal stage, vascular and nerve invasion, are also relevant for cancer staging.[26]
Methods of resection include local procedures such as polypectomy, mucosal excision or submucosal dissection and transanal endoscopic microsurgery. More invasive procedures include transabdominal resection, low anterior resection, proctectomy and coloanal anastomosis, abdominoperineal resection. [25-26]
Cure of disease:
The possibility of early diagnosis of cancer has led to a great reduction in the risk of cancer-related death, the term cured refers to complete clinical remission of the cancer. Long-term survivors of colon carcinoma account for 9.5% and rank third among all cancers.
4. Post-operative follow-up algorithm for colon and rectal cancer
* Article Authors
