Gastroenterology and Hepatology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2836-2888 | Journal DOI: 10.61148/2836-2888/GHR
Lázaro Antonio Arango Molano MD, FASGE1, Ileana Rocío Bautista Parada, MD2*
1General Surgeon, Clinical-Surgical Gastroenterologist, Universidad de Caldas, Manizales, Colombia
2General surgeon, Clinical-Surgical Gastroenterology Fellow, Universidad de Caldas, Manizales, Colombia.
*Corresponding author: Ileana Rocío Bautista Parada, General surgeon, Clinical-Surgical Gastroenterology Fellow, Universidad de Caldas, Manizales, Colombia.
Received date: December 09, 2021
Accepted date: December 22, 2021
Published date: January 05, 2022
Citation: Arango Molano LA, Bautista Parada LR. (2022) “Risk Factors and Screening Strategies for Early Detection of Esophageal Carcinoma.”, J of Gastroenterology and Hepatology Research, 3(1); DOI: http;//doi.org/01.2022/2.10126
Copyright: © 2022 Ileana Rocío Bautista Parada. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited
Esophageal cancer is the 8th most frequent type of cancer in the world and the 6th cause of death from cancer, with variable distributions according to geographic region, it´s mortality rate remains high because is rarely diagnosed at early stages. It has been reported that nearly 40% of patients have metastasized at the time of diagnosis; this added to the special anatomical conditions of the mediastinum and the high recurrence rate, confers it a worse prognosis and 5-year survival rate close to 20%. Initiatives for early detection, treatment, control and monitoring of different pathologies has shown a significant impact on reduction of morbidity and mortality in different pathologies, although there are no established protocols for esophageal cancer screening in general population, risks factors associated with development of this condition are well defined, and this allows identify and prioritize population with highest risk.
Introduction
Esophageal cancer is the 8th most frequent type of cancer globally and the sixth cause of cancer-related mortality, with variable distributions according to geographic region [1]. The most frequent histological type is squamous cell carcinoma (SCC), accounting for 87% of cases. However, in the United States and other western countries, adenocarcinoma (EAC) predominates, with a prevalence of 31% and 64% for SCC and ACE, respectively [2]. EAC has a more prolonged survival than SCC, especially if it is detected early; it is usually related to Barrett's esophagus (BE) and is located mainly at the distal third of the esophagus and / or at the gastroesophageal junction. BE increases the risk of EAC by 30-40 times. SCC is predominantly located in the upper two-thirds of the esophagus [3].
The onset of esophageal cancer symptoms is insidious and therefore can be confused with various pathologies including benign esophageal pathologies (such as motility disorders), thoracic, systemic or maxillofacial pathologies [4]. As in most gastrointestinal tumors, esophageal cancer predominates in men, accounting for 70% of cases. Men have a 3 to 4-fold higher risk than women of developing SCC and a 7 to 10-fold higher risk of developing ACE (1). The incidence is proportional to age, with 60% of cases are reported in patients older than 65 years and only 13% in those younger than 55 [5].
Esophageal cancer has a high mortality rate mainly because it is rarely diagnosed at early stages. It has been reported that nearly 40% of patients have metastasized at the time of diagnosis; this added to the unique anatomical conditions of the mediastinum and the high recurrence rate, confers it a worse prognosis and 5-year survival rate is close to 20% [6]. In localized disease survival is estimated in 30 months, with regional or distant extension, survival is estimated in 13 and 6 months respectively [7].
Initiatives for early diagnosis, treatment and monitoring of different pathologies have decreased morbidity and mortality. Although there are no established protocols for esophageal cancer screening in the general population, risk factors for this condition are well defined, identifying and prioritizing the population at highest risk. This review aims to describe the risk factors associated with the development of esophageal cancer and the best care for this group of patients.
Risk factors
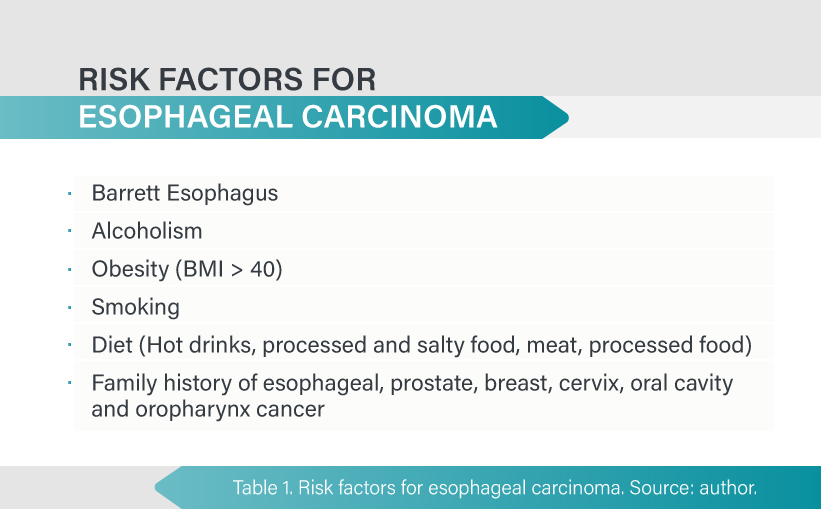
Esophageal cancer is usually preceded by chronic inflammation that impairs signaling and cell growth pattern. Alcohol consumption has been shown to increase the risk of SCC and BE and is considered the most important risk factor for EAC. Moreover, low intake of vitamins A and C, zinc deficiency, hot drinks, and infections such as human papillomavirus also increase SCC incidence [8]. There is conflicting data about family history of esophageal cancer as a risk factor for this disease. Studies performed in China, a country with a high incidence of SCC, have shown that patients who have a first-degree relative with SCC have double the risk of SCC [9]. Furthermore, family history of lung, prostate, breast, cervical, oral cavity and pharyngeal cancer also demonstrated an association with SCC [10].
Obesity is a known risk factor for EAC. A body mass index greater than 40 increases therisk twofold [11]. Alcohol and smoking are well established risk factors for SCC and some studies have shown a synergic effect. It has not been proven that alcohol increases EAC risk [12].
Regarding diet there is a protective effect from antioxidant properties of fruits and vegetables. In contrast, intake of hot drinks, meat, processed and salty food has shown an increased risk of developing esophageal cancer [13]. Table 1.
Barrett's esophagus
Adenocarcinoma is the predominant form of esophageal cancer in developed countries. Its incidence has increased six times in the last 40 years and has a poor prognosis with mortality rates almost equal to its incidence. Half of the patients opt for palliative care; however, patients with early stages have better outcomes, mainly associated with new endoscopic techniques [14].
Risk factors
Chronic inflammation of the esophagus is caused by gastroesophageal reflux or other irritants. Intestinal metaplasia (Barrett esophagus) may develop from this inflammation at the distal esophagus. Persistent irritation can lead to low-grade dysplasia, which can progress to high-grade dysplasia and later to adenocarcinoma [2].
Clinical risk factors for BE
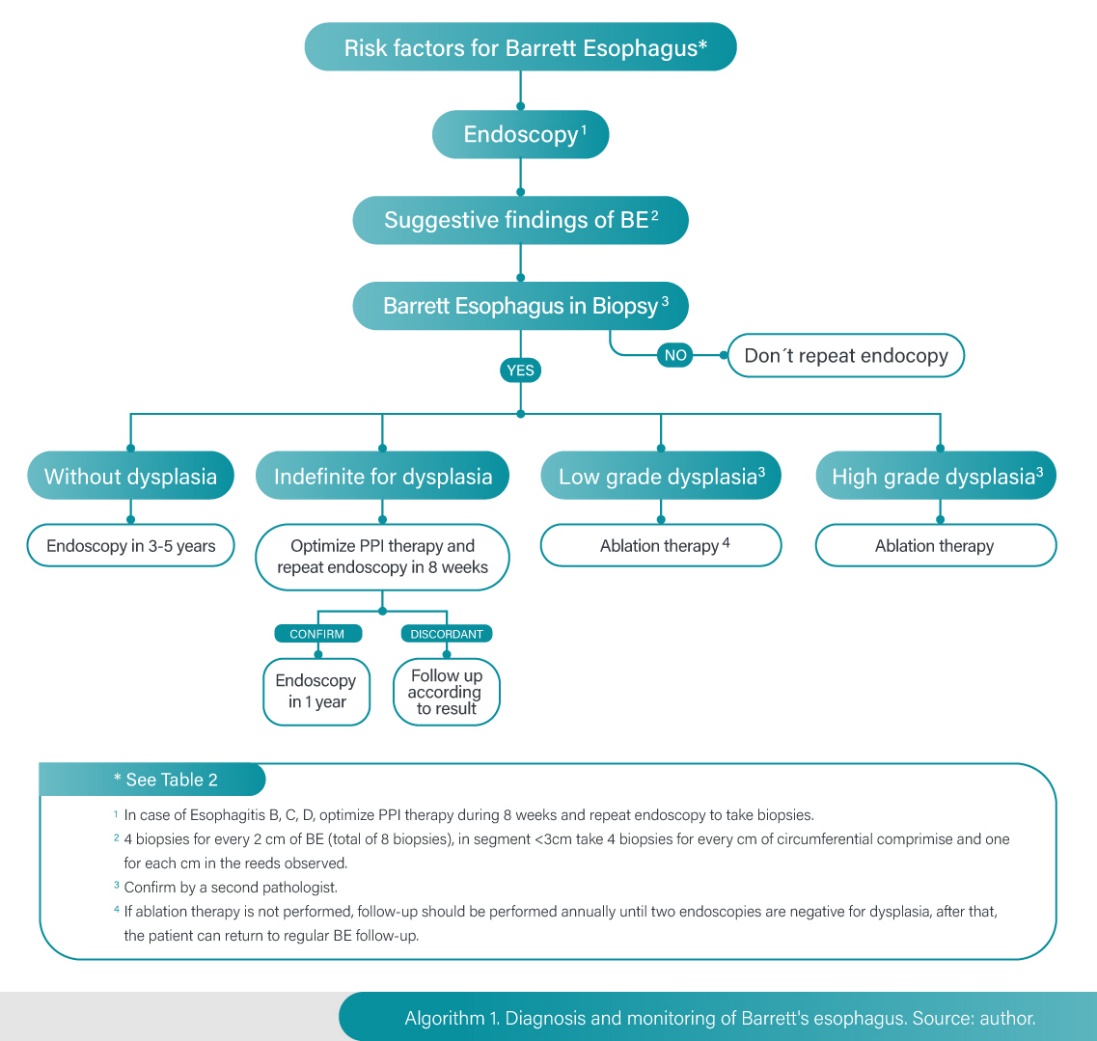
Although gastroesophageal reflux disease (GERD) is the strongest risk factor, 15-45% of BE cases develop in patients without GERD symptoms. Moreover, 15-20% of the western population have GERD symptoms, but only 10-15% develop BE. BE progresses to adenocarcinoma at a rate of 0.12 to 0.6% annually [15].
Screening for BE is recommended for men with GERD for over five years and / or symptoms more than once a week, with two or more risk factors for BE or EAC (age > 50 years, central obesity, abdominal circumference greater than 102 cm, tobacco use, first-degree relative with BE history) [16].
Follow-up is not recommended in women with chronic GERD symptoms, considering the low risk of EAC in this population. However, it can be considered in patients with multiple risk factors such as age greater 50 years, central obesity, abdominal circumference > 88 cm, tobacco use, first-degree relative with EB or EAC history [16, 17]. Table 2.

Endoscopic risk factors
Evidence of esophagitis or anatomic risk factors that worsen reflux (hiatal hernia) have been associated with an increase in the progression of BE and EAC. In patients with endoscopic suspicion of BE not confirmed by pathology, it is recommended to carry out endoscopic assessment in 1 to 2 years since up to 30% of these patients will have a positive result for metaplasia at the second review [16].
The extent of metaplasia determines if it is short or a long segment. Short segment is defined as less than 3 cm and long segment greater than 3 cm. Long-segment BE patients are at increased risk for EAC. Moreover, mucosal nodularity, ulcers, and areas of stenosis have also been associated with EAC development [18].
Histological risk factors
Rate of progression to EAC increases about 1% per year for BE patients with low-grade dysplasia and 7% per year for those with high-grade dysplasia [16].
Screening strategies
Screening techniques can be divided into those that allow the identification of patients with Barrett's esophagus and techniques to identify within patients with Barrett's esophagus, those at higher risk of developing CEA, so that they can have heightened surveillance and treatment if needed. This requires performing endoscopy and biopsies.
As mentioned previously, screening is recommended for high-risk groups and not recommended for the general population.
If Barrett's esophagus is identified, it is recommended to take four biopsies every two centimeters of metaplasia extension for a total of eight biopsies, if the segment is less than three centimeters, during the initial examination it is suggested to take four biopsies per centimeter of circumferential involvement. In patients with short-segment BE in whom it is not technically feasible to obtain eight samples, it is recommended to obtain at least four quadrant biopsies for each centimeter of circumferential involvement and one biopsy for each centimeter in the observed reeds [16,17].
If the initial endoscopy is negative for BE, it is not recommended to repeat it. If EVDA demonstrates grade B, C, or D esophagitis, endoscopic evaluation should be repeated after completion of 8-12 weeks of proton pump inhibitor therapy. To ensure healing and exclude the presence of underlying BE [16].
Follow-up
The use of advanced imaging techniques such as electronic staining during follow-up is recommended because it increases the detection rate of dysplasia by taking biopsies directed at any obvious mucosal irregularity. All mucosal irregularities, no matter how trivial they may appear, should be biopsied [19].
Dysplasia is the main risk factor for EAC in patients with BE, current evidence supports the importance of having confirmation by a second pathologist for all dysplasia reports (20).
The follow-up interval is determined by the presence and degree of dysplasia. Considering the low rate of progression to EAC of BE without dysplasia a follow-up of three to five years seems to be adequate in these patients. The protocol in this case requires biopsy from four quadrants at two-centimeter intervals [16].
If the report is indefinite for dysplasia, it is considered reasonable to optimize therapy with proton pump inhibitory therapy (PPI) at double dose to reduce any type of inflammation, it is suggested to perform endoscopic control in three to six months, if it is confirmed as indefinite, control is done after one year, and if it changes to high grade dysplasia (HGD) or low-grade dysplasia (LGD) or without dysplasia, the follow-up will be done according to this result [21].
If LGD is reported is recommended to confirm by a second pathologist, some authors suggest performing a new endoscopy after acid suppression with PPI in three to six months based on some studies in which up to 50% of these patients may have a change in the pathological report, this new endoscopic evaluation should be performed using chromoendoscopy that allows discarding with high precision the presence of lesions that require early mucosal resection (22). If LGD is confirmed, two protocols can be followed: follow-up or ablation therapy [16].
If no endoscopic therapy is performed because of patient's decision or conditions contraindicating it, follow-up should be performed annually until two endoscopies in a row are negative for dysplasia, after that, the patient can return to standard BE follow-up. The biopsy protocol in this case (follow-up of LGD) is to biopsy the four quadrants for each centimeter compromised by BE and any mucosal irregularity evidenced should be resected [16].
If high grade dysplasia is reported, it should be confirmed by a second pathologist and therapeutic intervention is required. Some authors suggest performing a new endoscopy in six to eight weeks to evaluate the presence of visible lesions that require endoscopic resection before ablative therapy [2,16]. Algorithm 1.
Treatment
in high-grade dysplasia, ablative treatment is preferred over esophagectomy or close endoscopic follow-up because of its proven efficacy and better safety profile compared to surgery. In BE with HGD (confirmed by two pathologists) ablative therapy results in clinically and statistically significant reduction of progression to HGD or EAC. In contrast, in EB without dysplasia (considering its low progression rate, the complication rate of ablative therapy and its costs), it is not recommended [22].
A wide variety of modalities have been described and shown to be effective to eradicate intestinal metaplasia. Currently photodynamic therapy and radiofrequency have the most valid evidence for reducing the incidence of EAC. Considering costs and safety profile of photodynamic therapy, as well as the data supporting safety and efficacy of radiofrequency, this appears to be the modality of choice for most patients. Stenosis and complete eradication rates for photodynamic therapy and radiofrequency are 30% vs. 6% and 77 vs. 90%, respectively [23].
Post treatment follow-up
After complete eradication of metaplasia, recurrence can be 8-10% per year and higher than 20% at two or three years. Most recurrences do not have dysplasia but nearly 25% may have it. The recurrence rate is similar for all ablation therapies. Endoscopic follow-up in patients with HGD history must be done at third, sixth, twelfth month and then every year. Endoscopic follow-up in patients with LGD should be done at first and third year and then every two or three years [16,22]. Algorithm 2.
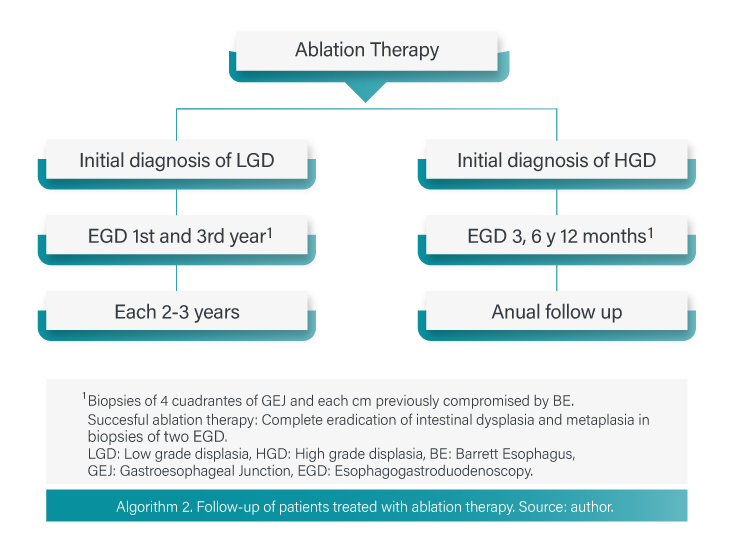
Is not recommended to discontinue the monitoring as long-term recurrences have been documented. Factors that favor recurrence are: age, large hiatal hernia and high-grade dysplasia before ablation.
Successful ablation therapy is defined as complete eradication of dysplasia as well as intestinal metaplasia in the esophagus, to confirm this biopsy of four quadrants of GEJ and from each centimeter previously compromised by BE are required. Two negative biopsy evaluations are required to consider complete eradication [17].
Squamous cell carcinoma
Although it is not the most common type of esophageal carcinoma in Western countries, ESCC remains the most prevalent type of esophageal carcinoma in the world.
Risk factor's
The most important risk factors for SCC are environmental. Smoking increases risk of developing the disease. Alcohol is another important risk factor with a relative risk increase of 2 to 8, depending on the volume of alcohol consumed. Consumption of food rich in nitrogenous components has an OR of 2 for development of this condition [2].
Screening techniques
Esophageal squamous dysplasia is believed to be the precursor lesion of ESCC; however, there are no prospective studies that confirm this theory. Chromoendoscopy is a fundamental tool in the diagnosis of pre-neoplastic squamous lesions [2]. The sensitivity and specificity of white light for the detection of high-grade dysplasia and cancer is 62% and 79%, respectively, compared to a much higher sensitivity of 96% when chromoendoscopy is used [24].
Due to the low incidence in Western countries, the screening strategy is not clearly effective, however, these are applied in regions of high incidence (30 cases per 100,000 persons/year) [25]. Two endoscopic strategies have been established that can be cost-effective. In areas with low-income levels and limited access to the health system, researchers recommend screening endoscopy at age 50, with follow-up every 5 years for low-grade dysplasia and every 3 years for high-grade dysplasia. In developed countries, 3 screening endoscopies are recommended at 5-year intervals starting at age 40 [2].
Early esophageal cancer
One-fifth of patients with esophageal carcinoma are diagnosed with localized disease incidentally during screening endoscopy or follow-up for other conditions. White light inspection may miss some lesions and therefore evaluation using chromoendoscopy and performing targeted biopsies of identified lesions is recommended; this strategy has demonstrated sensitivity > 90% and specificity > 80% for detection of high-grade dysplasia and early esophageal adenocarcinoma [26].
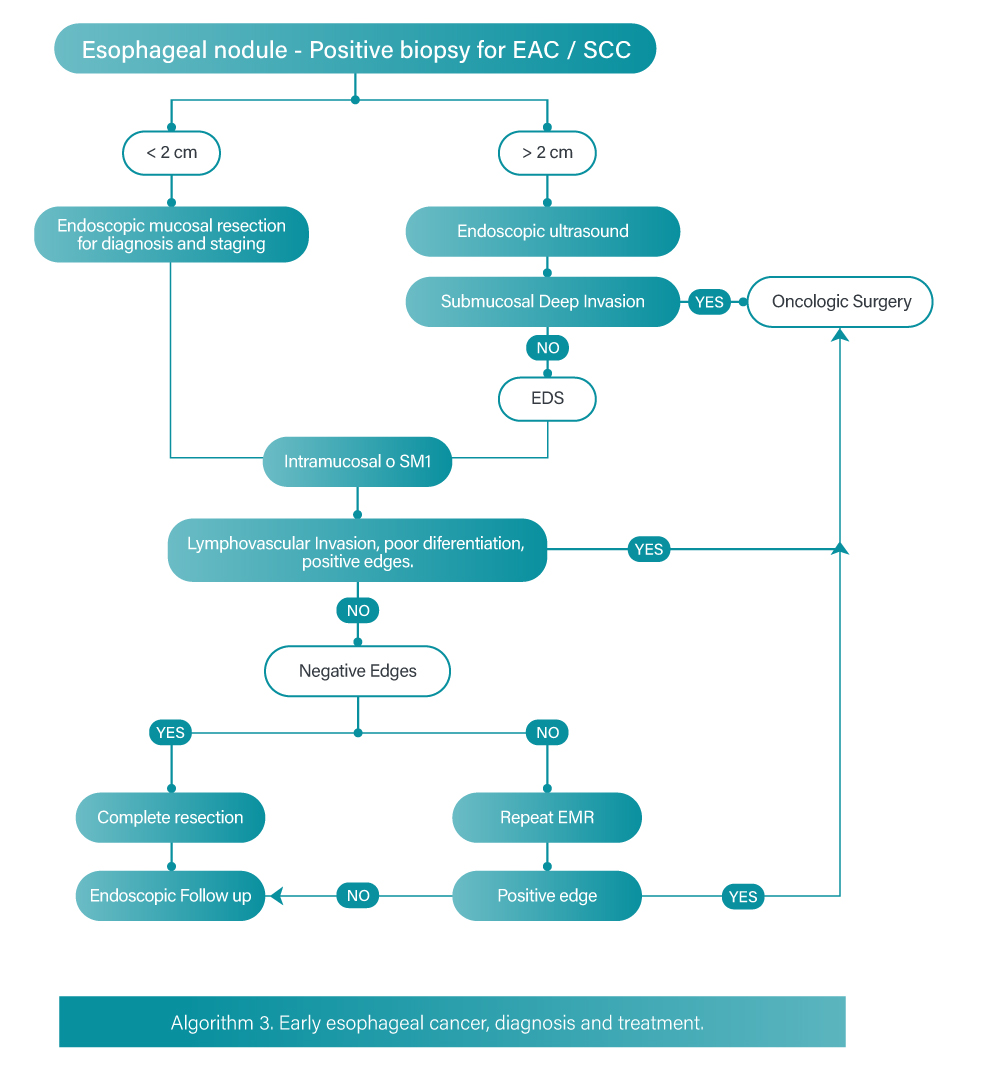
The cornerstone of early esophageal cancer is endoscopic resection. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are methods of endoscopic resection that allow the depth of invasion to be established. Endoscopic resection also provides information about differentiation degree and lymphovascular invasion [27].
Patients with short segments of nodular dysplasia and superficial lesions of adenocarcinoma or squamous cell carcinoma are amenable to endoscopic management by endoscopic mucosal resection. Endoscopic submucosal dissection has similar indications with the advantage of providing a deeper resection and therefore performing an en bloc resection with curative results; however, submucosal dissection is associated with longer operative time and a greater number of complications. Endoscopic dissection is indicated in lesions larger than 15 millimeters to achieve en bloc resection [28].
Endoscopic eradication therapy can have complete eradication rates R0 > 90% for T1a esophageal carcinomas, therefore it is recommended over surgery for carcinoma in situ and T1a for adenocarcinoma and squamous cell carcinoma.
Studies have shown that endoscopic resection is effective in eradicating high-grade dysplasia or T1a adenocarcinoma in 91-98% of cases. There are data suggesting that cure and survival rates after endoscopic resection of T1a stages are comparable to those reported after surgical treatment, but with much lower procedure-related morbidity and mortality [29].
T1b EAC lesions have a risk of lymph node metastasis as high as 15-25%. This risk can vary depending on the depth of submucosal invasion, with minimal risk of lymphovascular invasion for lesions limited to the upper third of the submucosa (Sm1); invasion beyond this segment is a predictor of nodal metastatic involvement and could then be a contraindication for endoscopic resection. Endoscopic resection is an option when dealing with T1b/SM1 lesions without high-risk features (poorly differentiated grade 3 or 4 carcinoma, lymphovascular or perineural invasion and deep positive margins) [26].
Despite aforementioned advantages, complications are also more frequent, including bleeding in 6.7% of cases and perforation in 4.6% [30].
Endoscopic ultrasonography performed before any treatment is important for the initial staging of neoplastic disease, as it provides information on depth invasion (T), lymph node involvement (N) and occasionally metastasis (M) [28]. Algorithm 3
Conclusions
Esophageal cancer is a pathology with increasing incidence in recent years, it has a poor prognosis with high mortality, mainly due to the fact that it is rarely detected in early stages and to the anatomical complexity of the mediastinum. There are clearly established risk factors for the development of this condition and the detection and close follow-up of this group of patients could have an impact on the course of the disease.