
Yacoub. Mkheittirate1*, Nizigiyimana Jean Marie1, Naouar Ouattassi1, Oudidi Abdelatif1, Najib Benmansour1 , Mohamed Ridal1 , Zaki Zouheir1, Hammas Nawal2 and Mohammed Nouredine EL Alami El Amine1
1Department of ENT Head & Neck & Maxillofacial Surgery, Hassan II University Hospital, Morocco.
2Anatomy, Surgery and Anesthesiology Laboratory, University of Sidi Mohammed Ben, Morocco.
*Corresponding Author: Yacoub. Mkheittirate, Department of ENT Head & Neck & Maxillofacial Surgery, Hassan II University Hospital, Morocco.
Received Date: April 18, 2024
Accepted Date: May 06, 2024
Published Date: June 18, 2024
Citation: Yacoub. Mkheittirate, Nizigiyimana Jean Marie, Naouar Ouattassi, Oudidi Abdelatif, Najib Benmansour, Mohamed Ridal.et.al. (2024) “ Acute Invasive Fungal Facial Cellulitis Associated to Actinomycosis in A Diabetic Patient : A Case Report.”, Clinical Medical Case Reports and Case Series, Head, and Neck Surgery, 1(1); DOI: 10.61148/ CMCRCS/001.
Copyright: © 2024. Yacoub. Mkheittirate. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction:
Acute invasive fungal cellulitis is a potentially leathal infection due to opportunistic fungi of the order Mucorales, mainly affecting immunocompromised individuals. Due to its non-specific symptomatology, dominated mostly by tissue necrosis, diagnosis is often delayed, adversely affecting prognosis. This infection predominantly arises in the nose and paranasal sinuses. Mucormycosis affecting sollely the cheek region are uncommon. We believe this ist he first case of localized mucormycosis of the cheek. Moreover, Actinomycoses and mucormycoses occur most often in patients with immunodepression, and their association is less well described in the literature.
Case Presentation:
We present the clinical, biology and imaging findings of a rare case of jugal mucormycosis associated with actinomycosis in a 56-year-old female patient who presented with unbalanced diabetes and discuss diagnostic and therapeutic challenges.
Discussion & Conclusion:
Mucormycosis and actinomycosis are rare infections that occur most frequently in immunocompromised patients, Mucormycosis is caused by the Rhizopus arrhizus species. The cutaneous localization of mucormycosis is uncommon and is favored by a breach, be it a burn, a catheter or any other cutaneous trauma. Cervicofacial Actinomycosis occurs in over 60% of cases in the maxillofacial region. It is the anatomopathological examination which gives a diagnosis of certainty of these two pathologies and their late diagnosis is coupled with serious complications.
fungal cellulitis; infection; Mucorales
Introduction:
Mucormycosis is an opportunistic fungal infection caused by fungi of the order Mucorales. [1] The first case of mucormycosis was reported by Paultauf in 1885. [2] These infections are generally observed in immunocompromised individuals, usually with uncontrolled diabetes mellitus, hematological malignancies such as leukemia, or patients under immunosuppressive therapy. [1] Individuals suffering from burns or extensive traumatic wounds are a population at risk of such infections, whose spore transmission vectors may include rain, wind, insects, ventilation systems, dressing materials or handling. [3 ,4] Filamentous fungal infections include those caused by germs of the Mucorales, Aspergillus and Fusarium genera. The germs of the Mucorales genus responsible for mucormycosis are Rhizopus, Mucor, Lichteimia and Rhizomucor [5]. On the basis of clinical presentation and anatomical site, mucormycosis can be divided into at least six clinical categories: rhino-orbito-cerebral (44%-49%), followed by cutaneous (10%-19%), pulmonary (10%-11%), disseminated (6%-11%), gastrointestinal (2%-11%) and miscellaneous. However, the definitive diagnosis of mucormycosis can only be made by biopsy (which identifies the characteristic hyphae) and by fungi culture [7]. Actinomycosis is a specific, rare, non-contagious bacterial disease that preferentially affects the cervicofacial sphere. [8] It is a subacute to chronic infection caused by the Gram-positive filamentous non-acidic anaerobic to microaerophilic bacterium Actinomyces. Approximately 70% of infections are due to either Actinomyces israelii or Actinomyces gerencseriae. [9] Actinomyces normally colonize the human mouth, urogenital tract and gastrointestinal (GI) tract, but under certain circumstances they can become pathogenic. [8 ,7 ,9] Actinomycosis and mucormycosis are rare infections that occur most frequently in immunocompromised patients, and the association of these two pathologies is rarely reported in the literature. [1,10] We present a case of acute invasive fungal jugal cellulitis with actinomycosis superinfection.
Case Report:
We report the case of a 48-year-old unbalanced diabetic patient who presented to the ORL outpatient clinic for an apyretic right jugal cellulitis evolving for 12 days. Local clinical examination revealed a painful jugal infiltration on palpation, with no local heat or swelling or trismus (figure1). Oral cavity examination revealed poor oral hygiene, with decayed teeth and pus draining from ulcerations on the inner surface of the left cheek. We suspected cellulitis, and pus sampling with routine blood tests were ordered : CBC: WBC 14130/mm 3 NNP 11047/mm 3 HB 13.30 g/DL PLT 490,000/mm 3 C-reactive protein 170.80 mg/l fasting blood glucose 5.17 g/l, negative ketonuria; HBA1c 10%. Analysis of the pus was positive for Actinomycosis. Cervical-facial CT revealed extensive infiltration of the right jugal soft tissue, extending to the masseter muscle and infra-temporal fossa, with no clearly visible collection suggestive of cellulitis (Figure 2). The patient received first a probabilistic antibiothérapy with amoxicillin and clavulanic acid (80mg/Kg per day) adjusted 03 days later according to the antibiogramme that reveiled an actinomyces israelii Susceptible to Penicillin and Clindamycin. After 10 days of antibiotics we didn’t noticed any clinical or laboratory improvement. CBC: WBC 17130/mm 3 NNP 14047 / mm 3 HB 12 g /DL PLT 400 000/mm 3 C-reactive protein 200mg/l fasting blood glucose 3.17 g/l, ketonuria negative. A biopsy was then taken, and histological examination revealed fatty tissue remodelled by polymorphic inflammatory infiltrates consisting of lymphocytes, plasma cells and neutrophils, some of which were altered; presence of necrosis; presence of PAS-positive mycelial filaments. The presence of pathogenic agent in the form of rosettes was also noted, all pointing to mucormycosis associated with actinomycosis (figure 3). The patient was then put on systemic amphotericin B 5mg/Kg/day with clinical and biological improvement after 7 days of treatment with no need of surgical debridement.
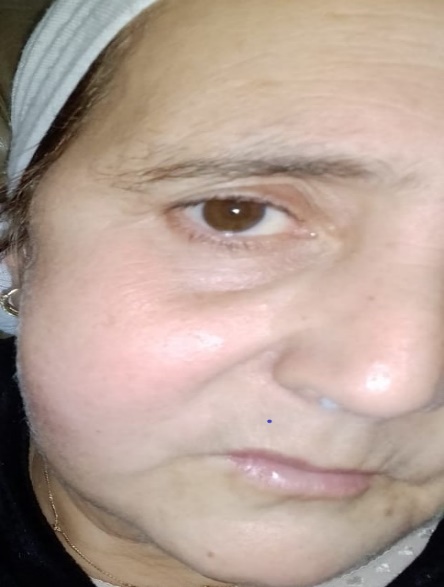
Figure 1: Image showing infiltration of the right jugal skin
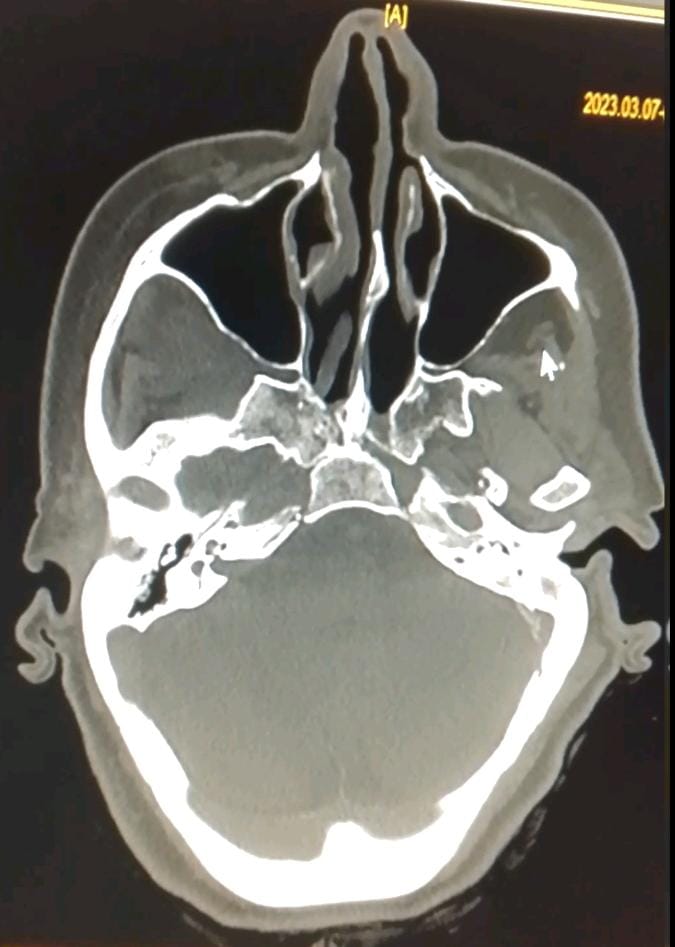
Figure 2: CT image showing infiltration of the jugal soft tissues, with integrity of the bone structures opposite.
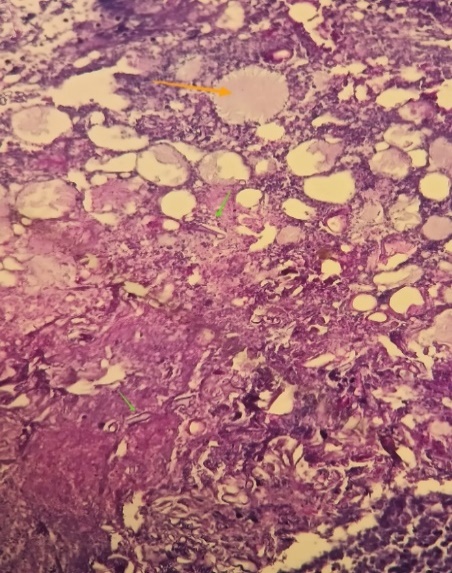
Figure 3: Presence of mucormycosis filaments (green arrow) with actinomycosis
Discussion:
Mucormycosis and actinomycosis are rare infections that occur most frequently in immunocompromised patients, and the association of these two pathologies is rarely reported in the literature. [1,10] Mucormycosis is the third most common invasive mycosis caused by fungi of the Zygomycetes class. [11] The mortality rate of disseminated mucormycosis in burn patients is estimated at up to 50% in the literature and up to 20% in the general population. [4.7] On the basis of clinical presentation and anatomical site, mucormycosis can be divided into at least six clinical categories: rhino-orbito-cerebral (44%-49%), followed by cutaneous (10%-19%), pulmonary (10%-11%), disseminated (6%-11%), gastrointestinal (2%-11%) and miscellaneous. [18] Mucormycosis is caused by the Rhizopus arrhizus species. The cutaneous localization of mucormycosis is uncommon [19]. The cutaneous localization is favored by a breach, be it a burn, a catheter or any other cutaneous trauma, especially when the skin is covered with bandages and more particularly elastic adhesive strips [20]; bandages in fact favor maceration and proliferation of the fungus, due to the occlusion. Rhizopus has a particular tropism for skin covered with occlusive bandages. In our case, it was an isolated mucormycosis oft he cheek, as there was no breach or other trauma to the skin. Two types of primary cutaneous mucormycosis have been described: a subacute superficial form characterized by vesicles or pustules that progress to bedsores in an otherwise normal host, and a rapidly progressive gangrenous form in patients with impaired immunity. It is characterized by a central black necrotic zone bordered by erythematous to purplish cellulitis, and by the potential for dissemination in the absence of treatment. [15,16] Although cutaneous disease is generally considered a benign form of zygomycosis, necrotizing primary cutaneous lesions with subsequent dissemination have been observed in severely ill children. [17,18] In our patient, there was no skin necrosis, as the diagnosis was made at an early stage.
Histologically, mucormycosis is characterized by extensive tissue necrosis and the presence of numerous large (5 to 30 μm), thin-walled, non-partitioned, right-angled branching fungal hyphae with a ribbon-like appearance. [19,20] There is a close histopathological resemblance between the fungal hyphae of mucormycosis and aspergillosis. The distinguishing feature is that the hyphae of mucormycosis are non-septate and branch at right angles, whereas the hyphae of aspergillus species are septate, smaller in width and branch at more acute angles. [21]
Successful treatment of mucormycosis involves rapid and accurate diagnosis of the condition, followed by radical surgical debridement of infected necrotic tissue and systemic administration of antifungal drugs [22]. The most commonly used drug is amphotericin B deoxycholate, with a cure rate of 80% [23]. A new triazole derivative, pasaconazole, an oral antifungal agent, has recently been used, either alone or in combination with amphotericin B. The underlying systemic disease must be checked immediately [22]. In our patient, treatment was medical with amphotericin B 5mg/Kg per day.
Aside from the atypic localization oft he mucormycosis, our patient presented with actinomycosis secondary infection. Actinomycosis is a rare (1/300000 inhabitants) and serious infection, sometimes life-threatening if diagnosed late. This condition is described in several locations: cerebral and cervico-facial (50-70% of cases), thoracic (15-20%), abdominal and pelvic (10-20%). [24] Cervicofacial Actinomycosis occurs in over 60% of cases in the maxillofacial region. Half of all cases involve the tissues surrounding the toothed portion of the mandible, followed by the jugal region (15%), the chin (15%), then the ascending ramus and mandibular angle (10%). More rarely, the temporomandibular joint may be involved. In less than 3% of cases, cervico-facial Actinomycosis may manifest as macroglossia [9]. Exceptional localizations have been described in the sinuses, middle ear, external ear, larynx, lacrimal tract, orbit, thyroid gland and central nervous system [10]. Actinomyces normally colonize the human mouth, urogenital tract and gastrointestinal (GI) tract. As a commensal organism, it is very difficult to determine normal flora colonization versus infection. [9,8] In 70% of cases, A. israelii and Actinomyces gerencseriae species are involved. [9] The disease occurs in subjects with poor oral hygiene and/or a history of oral trauma (in particular, tooth extraction) or an immunity impairement (immunodepression, corticosteroid therapy, diabetes, neoplasia, etc.). Diagnosis is often delayed due to the clinical polymorphism of the disease and the difficulty of identifying the germ. The clinical picture is characterized by a slowly progressive, painless swelling of the cheek, with indurations followed by subcutaneous fibro-cicatricial remodeling. [19] This syndrome is known as lumpy jaw. If it becomes chronic, abscesses with fistulas may develop with endobuccal or extraoral drainage. If left untreated, cervico-facial actinomycosis can extend deep into the body, invading bony structures. Positive diagnosis is based on bacteriological examination of the pus collected from the lesion, which confirms the diagnosis by demonstrating the presence of filamentous gram-positive bacilli. However, isolation and identification of the bacterium are uncommon (less than 50% of cases due to prior antibiotic prescription or inadequate methodology). [25] The diagnosis of this condition is most often made by anatomopathological examination of the sampled material, which identifies the actinomycotic granule. Treatment is medical, sometimes supplemented by surgery [24]. The antibiotic of choice is penicillin. The role of surgery in the treatment of actinomycosis is limited to drainage of fistulizing forms, removal of encysted pseudotumoral forms not controlled by medical treatment, removal of bone sequestration and excision of necrotic tissue [22].
Conclusion:
In summary, we present a complicated association of two uncommon diseases. Mucormycosis is a rare invasive fungal disease which exceptionnaly occurs in the cheek which may delay the diagnosis and cause serious, sometimes fatal, complications. Secondary infection of mucorcmycosis with actinomycosis is uncommon, but important to consider in everyday practice before a case of recalcitrant cellulitis. It is therefore essential to understand these infections, as their late diagnosis is coupled with serious complications.