Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 12 - Issue 1 - 2025
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
S.Bennaleg- L.Atroune- N.Habchi- K.Bouaita- M.Djaafer
Department of Neurosurgery Mustapha Bacha hospital Algiers, Algeria
*Corresponding author: H. Bekralas, Department of Neurosurgery Mustapha Bacha hospital Algiers, Algeria.
Received: March 20, 2021
Accepted: April 09, 2021
Published: April 19, 2021
Citation: S.Bennaleg- L.Atroune- N.Habchi- K.Bouaita- M.Djaafer, “ Brain Metastases in Hepatoblastoma A case report ”. Clinical Case Reports and Clinical Study, 3(3); DOI:10.61148/2766-8614/JCCRCS/047
Copyright: © 2021 H. Bekralas. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Brain metastases from hepatoblastomas are very rare. We report a case of an 11 months old female who developed multiple intracranial metastases and underwent surgery. Only 39 patients were identified in literature and it was indicated that brain metastases are a fatal complication of hepatoblastoma. In a child with known hepatoblastoma, with the development of neurologic signs or symptoms, an imaging study of the brain, preferably a magnetic resonance imaging should be performed to exclude the presence of cerebral metastases.
Introduction
Hepatoblastoma (HB) is the most common primary malignant liver tumor in infants and young children (1, 2, 3, 4). The incidence in children age 4 or younger is 3.8 per million (5, 6). Tumor metastasis is the principle obstacle to the development of efficient treatments for patients with HB; they usually affect the lung; brain metastases are exceedingly rare. Although the overall survival for children with HB is now approaching 80%, 1 those who relapse are a challenge, especially those with non pulmonary recurrence; 39 patients were identified in literature (7), our patient would be the 40th.
An 11-month-old female presented with an abdominal distention. Physicians observed a large abdominal mass. A computerized tomographic study of the chest, abdomen, and pelvis; and abdominal ultrasound was performed identifying a single mass enlarging the liver. The serum alpha-fetoprotein was at 100,000 ng/mL. A percutaneous liver biopsy confirmed the clinical impression of a standard risk hepatoblastoma according to Siopel classification. The patient received phase A of chemotherapy with two cycles of cisplatin then three cycles with cisplatin and adriamycine; then phase B with carboplatine and doxorubicine. Afterwards, the patient underwent complete resection of the residual tumor. Finally our patient received phase C of chemotherapy with doxorubicine and carboplatine.
Eleven months after diagnosis, faced with persistent elevated serum alpha-fetoprotein, the patient received the ICE protocol of chemotherapy; during the hospitalization, our patient presented with episodes of tonic-clonic seizures. A computed tomographic scan of the head indicated a supratentorial mass, suspicious for superimposed leptomeningeal disease. The lesion was located in the right fronto-parietal lobe, causing regional edema and mass effect (Fig 1).
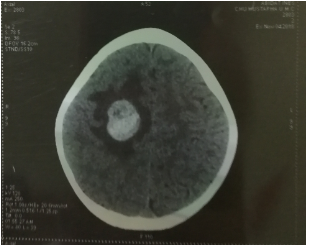
Fig 1: A computed tomographic scan of the head indicated a supratentorial mass.
Dexamethasone was begun, and the patient was transferred to our neurosurgery department, an MRI was performed objectiving An Axial T1-weighted image after contrast demonstrates an appearance of mixed hypointense, but also iso- and hyperintense areas corresponding to hemosiderin with a central nodular lesion in the right rolandic parietal lobe; enhancement was seen after contrast (Fig 2). Our patient underwent surgery; anatomopathological examination came in favor of metastasis of hepatoblastoma. During the postoperative chemotherapy the child complained of headache followed by several episodes of vomiting and irritability. A second MRI was performed objectifying 03 heterogeneous right rolandic, thalamic and occipital lesions (Fig 3). However, the patient had a cardio-respiratory arrest during the hospitalization. Resuscitation was not attempted.

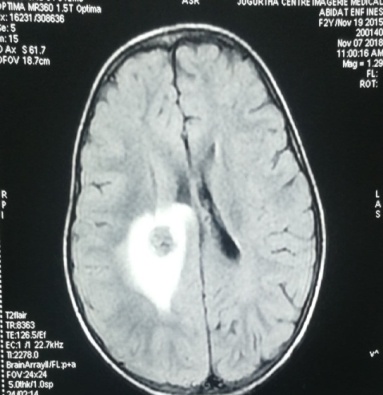
Fig 2: an MRI of 2 year old patient showing a central nodular lesion in the right rolandic parietal lobe.


Fig 3: an MRI of a 2 year old patient showing 03 heterogeneous right rolandic, thalamic and occipital lesions.
Discussion
Headaches in children with cancer, even when focal localizing signs are absent, often indicate structural brain disease and require an imaging study of the brain (8). When children develop brain metastases and the primary cancer is sensitive to chemotherapy, prolonged survival and even cure can sometimes be achieved (8). Brain metastases are exceedingly rare in children with hepatoblastoma; Twenty-seven publications cited actual cases (n= 39) of HB metastatic to the brain. Of the 38 patients in these reports, only 19 of them had clinical information included. A review of the literature is summarized in Table 1(7).

Dx indicates diagnosis; F, female; HA, headache; HB, hepatoblastoma; Lt, left; M, male; NS, not stated; Pt, patient; Rt, right.
|
5-FU indicates 5 fluorouracil; AFP, alpha fetoprotein; Carbo, carboplatin; CDDP, cisplatin; CNS, central nervous system; CR, complete response; Dx, diagnosis; Gy, gray; mo, month; NR, no response; NS, not stated; PD, progressive disease; PR, partial response; Sx, surgery; VCR, vincristine; VP16, etoposide; WB, whole brain; XRT, radiation therapy. |
Table 1 clinical information of 20 cases with Brain metastases
Median age at original diagnosis was 24 months with 4 cases being above 4 years old (10); and the stage at original diagnosis being stage IV in 4 patients similar to our case. Histological information was limited to only 10 patients. Alpha fetoprotein (AFP) data were also limited, with only 6 patients having levels reported, either at original diagnosis or at the time of brain metastasis. All of the AFP values were >100 ng/mL and elevated for age. The interval from original diagnosis to brain metastasis was 24 months median, while it was 12 months in our case. Regions of the brain reported as involved with metastases were occipital 5, parietal 5, frontal 4, and temporal 2. The side of the brain involved appeared equivalent: 10 lesions on the right similar to our case and 11 on the left. All patients had neurological symptoms at the time of diagnosis of brain metastasis. Evidence for any disease dissemination (at original diagnosis or recurrence) before the brain metastasis was found in most of the patients (7 of 11 with relevant information reported: all had pulmonary metastases alone); contrary to our case and the other four patients (patients 2, 3, 10 and 17) who had no evidence of distant metastasis before the occurrence of the brain metastasis. A single lesion was present in original diagnosis or recurrence) before the brain metastasis was found in most of the patients (7 of 11 with relevant information seven patients.
The other children had several metastases similar to our case. Multiple lesions are the rule in pediatric brain metastases, as is the presence of hemorrhage (26).

Details of the treatment and outcomes for 14 patients are given in Table 2 (7). Only 3 of them (patients #1, 5, and 10) were alive after 10 years, 2 years, and 6 months respectively. The treatments given to these patients were quite varied and included chemotherapy alone (5 cases); surgery and chemotherapy (2 cases) similar to our case; no treatment (2 cases); chemotherapy plus radiation therapy (2 cases); and 1 each for surgery plus radiation therapy, and surgery plus chemotherapy and radiation. Only 7 of the 13 patients had responses of the brain metastases to the treatment reported, while our patient had no response. It seems that the 4 patients, who received some radiation as part of their therapy survived 6 or more months, whereas only 2 of 6 patients who did not receive any radiation survived that long. Response was noted only in one patient: 56% reduction in tumor volume due to radiation therapy and temozolomide. (7)
Knowledge of the molecular mechanisms that causes metastatic spread in HB patients would help with approaches to clinically manage patients with metastasis according to their individual risk profile and with detection of metastases at an earlier stage. However, due to the rare occurrence of this tumor, there is only limited data available on the important players in metastatic HB; the transcription factor SP8 and the growth factor FGF8 were identified as two of the most strongly up-regulated genes in metastatic HB cases(27). High SP8 and FGF8 expression was associated with the presence of the aggressive C2 subtype of the 16-gene signature (28) and poor outcome, and could be linked in vitro to aggressive traits such as cell motility, self-renewal, migration, and invasion. Consequently, these effects could be successfully abrogated by down-regulating FGF8, which was identified as a direct transcriptional target of SP8 (27).
It was also demonstrated that elevated Thymosin β4 expression in HB cells may promote HB metastasis via the deregulation of EMT which consists of a coordinated series of stages, where epithelial cells lose their cell polarity and their cell-cell adhesion, and develop into mesenchymal cells, which results in tumor invasion and metastasis. The process of EMT is essential for HB metastasis and invasion to occur; it was found that Tβ4 expression was significantly higher in HB tissue cells compared with that in healthy adjacent cells. (29)
Surgery should be considered for single large hemorrhagic lesions even in chemosensitive tumors, given the potential for bleeding and the clinical advantage the surgery will give. In the current case, surgery was performed at first, and then excluded in the presence of multiple lesions; the sudden neurologic deterioration might be a result of acute bleeding. Cranial irradiation, the standard treatment for adults with multiple metastases, is in general avoided in children of young age; however, the dismal prognosis did not allow concerns regarding effects of radiotherapy. The only child with prolonged survival had a single recurrent cerebral metastasis, successfully treated with a combination of multiple surgical resections, chemotherapy, and radiation therapy (26).
Few, relatively old overviews of brain metastases in children have been previously reported and little is known about the changes that have come with the advent of aggressive multimodal therapy, such as intensive chemotherapy and high-dose chemotherapy with stem cell rescue. It appears that The CNS is a very rare metastatic site for HB, although it might be more common than realized due the lack of routinely CNS imaging when possible recurrence of HB is being investigated. Although the patient numbers is small, it seems like certain factors plays a role in the recurrence of this complication: patient age at original diagnosis, advanced disease at original HB diagnosis, and evidence of multiple prior pulmonary although the data was not enough to permit any conclusions about the importance of sex, race, tumor histology, or serum AFP. The treatments reported in previous studies were quite varied and nothing was notable, except a little advantage of use of radiation therapy that needs more investigating. At any rate, we feel that the data presented support a recommendation to evaluate the brain in any HB patient presenting with CNS symptoms at the time of a suspected recurrence as we suspect that dissemination of HB to the CNS occurs more often than previously thought.
Conclusion
If a child with a hepatoblastoma develops neurologic complaints, an imaging study of the brain, preferably a magnetic resonance imaging, should be performed to exclude the presence of cerebral metastases. Although in general the prognosis is poor, if a single lesion is detected, it may be possible to achieve long survival with aggressive, multimodality treatment.