
Tian zheng2, Qianwei Huang1,Xiao Huang1,Qianghui Huang1,Jianxin Hu1, Xiaoshu Cheng1, Biming Zhan1*
1 Department of Cardiology, The Second Affiliated Hospital of Nanchang University.
2 Department of Radiology, The Second Affiliated Hospital of Nanchang University.
*Corresponding author: Biming Zhan, Department of Cardiology, the Second Affiliated Hospital of Nanchang university.
Received date: August 22, 2023
Accepted date: September 30, 2023
published date: November 20, 2023
Citation: Zheng T, Huang Q, Huang X, Huang Q, Hu J, Cheng X, Zhan B, (2023) “Comparison of the Efficacy and Safety of Nifekalant in The Instant Cardioversion of Paroxysmal Supraventricular Tachycardia.”. journal of clinical cardiology interventions, 3(2); DOI: http;//doi.org/10.2023/07.1037.
Copyright: © 2023 Biming Zhan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Objective: The standard course of treatment for paroxysmal supraventricular tachycardia (PSVT) is intravenous antiarrhythmic medication. The goal of this trial was to determine whether prehospital administration of nifekalant for the treatment of PSVT was effective and safe.
Method: Data from 15 patients with PSVT who received prehospital first aid in hospital were collected retrospectively. Under continuous ambulatory monitoring, patients received one dose of 0.3 mg/kg nifekalant. The time to conversion to sinus rhythm and the longest QTc interval were all recorded for all patients in the acute care area.
Results: Within 30 minutes, nifekalant had a PSVT conversion efficacy of 60%. Torsades de pointes and ventricular fibrillation were not found. Other common drug-related adverse events, such as hypotension, heart failure exacerbation, dyspnoea, and chest pain, also did not occur. No serious Nifekalant-related adverse events or deaths were reported.
Conclusion Nifekalant is a safe and effective option for the treatment of PVST. A 0.3 mg/kg dose of intravenous nifekalant may be recommended for PSVT.
Introduction
Paroxysmal supraventricular tachycardia (PSVT), which consists of atrioventricular nodal reentrant tachycardia (AVNRT) and atrioventricular reentrant tachycardia (AVRT), is particularly frequent in the emergency department [1]. It is distinguished by a typical tachycardia with abrupt onset and termination [2]. The most frequent symptoms of PSVT are rapid heart rate, palpitations, light-headedness, shortness of breath, chest pain, anxiety, and possibly syncope [3-4].
PSVT discontinuation is the primary treatment goal in patients who cannot tolerate prolonged fast tachycardia and patients who are in a state of unstable haemodynamics [5]. Catheter-based radiofrequency ablation (RFA) of PSVT is highly effective, giving up to 85% of people symptom relief [6]. Pharmacologic treatment is more appropriate in the emergency department and includes intravenous adenosine and calcium antagonists.
Nifekalant is a novel classification III antiarrhythmic drug that inhibits the fast factor of delayed-rectifier potassium currents whilst permitting inward sodium and calcium currents to pass [7]. Nifekalant seems to be an effective remedy for refractory ventricular arrhythmia [8], and it has recently been used in the treatment of atrial arrhythmia. The success of nifekalant in the treatment of PSVT has not been studied [9].
We have found that nifekalant significantly prolonged the effective refractory period and the block cycle length of the antegrade accessory pathway but had no effect on the effective refractory period of the antegrade atrioventricular node, which could be a mechanism by which it could treat PSVT [10]. The purpose of this study was to evaluate the efficacy and safety of nifekalant in PSVT patients.
Methods
Study population
The study population consisted of consecutive patients with electrocardiography (ECG)-proven PSVT who were admitted to the Second Affiliated Hospital of Nanchang University between February 2021 and December 2022.
Before receiving any emergency cardioversion treatment, potential patients were screened for study enrolment. Patients with a systolic blood pressure greater than 90 mmHg, atrial fibrillation or atrial flutter, aortic stenosis, myocardial infarction, glaucoma, retinopathy, or a need for urgent cardioversion (e.g., pregnant or critically ill) were excluded.
Patients were randomly assigned to receive a single intravenous dose of nifekalant (50 mg, 0.3 mg/kg for 5 minutes, followed by a continuous infusion at 0.3 mg/kg per minute for the residual dosage; Sichuan Bai li Pharmaceutical Co. Ltd., Sichuan, China), followed by 120 minutes of observation. The conversion time to sinus rhythm (SR), the RR interval during conversion to SR, and the longest QTc interval were all recorded.
All patients were monitored continuously for two hours in the emergency department's acute care area. If the rhythm on the monitor changed, an ECG trace was immediately taken, and if the new ECG revealed a recurrence of PSVT, the patient was treated accordingly.
Any indication of a side effect, such as persistent bradycardia, hypotension, nausea, vomiting, headaches, dizziness, itching, breathing difficulties, chest pain, or flushing, was noted.
The primary outcome measure was the rate of maintaining SR within 2 h, and the secondary outcome was the occurrence of adverse effects of nifekalant.
Data analysis
Continuous variables with normal distributions are reported as mean±standard deviation and were compared by Student’s t test. We used Student’s t test or the χ2 test to compare means. All data analyses were performed with SPSS version 16 (SPSS, Inc., Chicago, IL, USA).
Results
Characteristics of study subjects
A total of 15 patients were eligible for the study. Their baseline characteristics are listed in Table 1. Most patients were women. Hypertension was the most common comorbidity. Palpitations were the major chief complaint.
Table1: Baseline characteristics of population
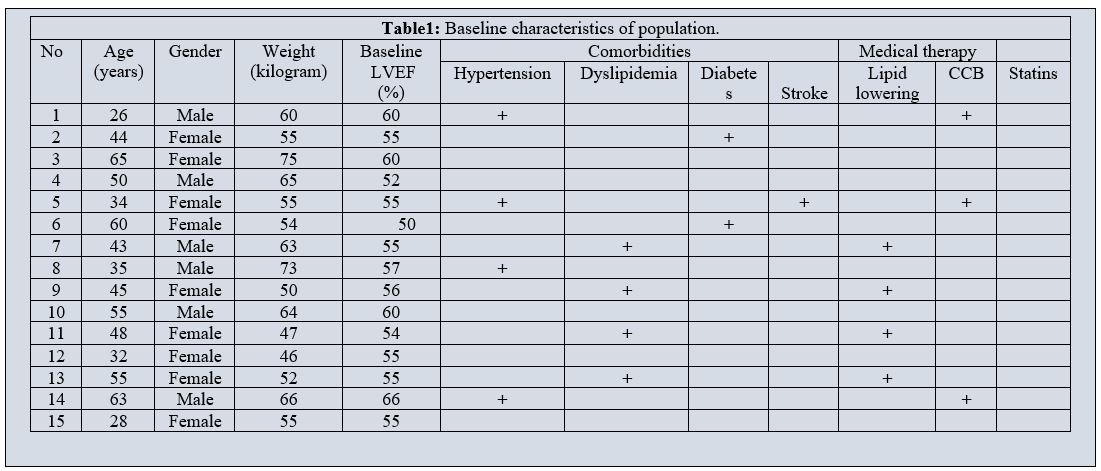 CCB, Calcium channel blockers
CCB, Calcium channel blockers
Main results
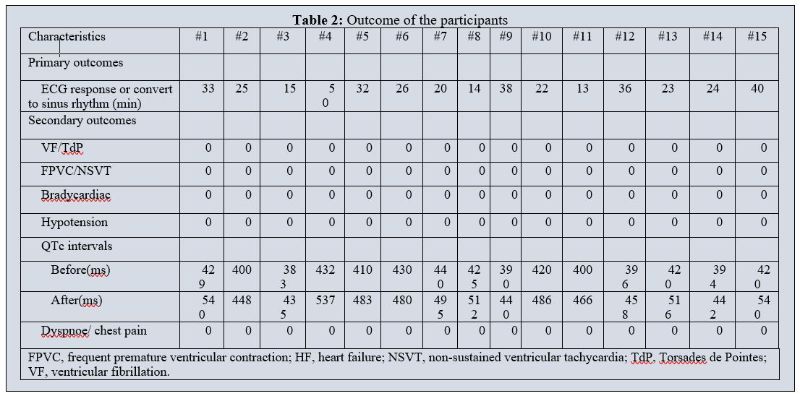
Table 2 illustrates the primary and secondary outcomes. The primary outcome, successful termination of PSVT, was achieved in 60% within 30 min, and the average conversion time was 27.4±10.24 min. In the primary safety analysis, torsade de pointes (TdP) and ventricular fibrillation were not found within 2 hours after infusion, nor were other relevant adverse reactions, such as hypotension, heart failure exacerbation, dyspnoea, or chest pain.
Table 2: Outcome of the participants
Discussion
In this study, we demonstrated that the pure class III antiarrhythmic agent nifekalant had potent PSVT conversion efficacy. A 0.3 mg/kg dose of intravenous nifekalant may be recommended during the conversion of PSVT.
PSVT has traditionally been considered a benign rhythm disorder, but recent studies have suggested that patients with PSVT may have a higher risk of ischaemic stroke, and early treatment aimed at symptom reduction is necessary [13]. The standard Valsalva manoeuvre (VM) is recommended as the first-line strategy for the termination of PSVT by many international guidelines[14]. Although the standard VM is safe, cost-free, and easily doable by nurses or doctors, the success rate of cardioversion is relatively low (5%-20%), and when these fail, pharmacological treatment is needed.
For many years, the mainstay of treatment for stable PSVT was adenosine. It binds to the adenosine A2 receptor in the atrioventricular node area, reversing atrioventricular node reentry conduction [15]. Although ATP conversion is effective, some patients will experience self-consciousness, suffocation, and syncope. Many patients report the transient effects of adenosine to be very distressing, and the potential for long-term psychological effects cannot be discounted [16]. The most common alternative to adenosine is verapamil, especially for those who have not been given adenosine. Due to the vasodilatory characteristics of beta-blockers, verapamil may cause flushing, dizziness, and a multiplied hazard of hypotension [17,18]. Another alternative drug is amiodarone. It is traditionally believed that amiodarone may lead to extracardiac side effects, such as pulmonary, thyroid gland, or liver abnormalities, vision changes, skin reaction, and nerve damage, especially in long-term therapy, which makes it a second-line treatment [19].
Nifekalant was approved in 1999 for the treatment of ventricular tachyarrhythmias. It does not block sodium or calcium channels and has no effect on myocardial contractility [20]. Therefore, its incidence of adverse events, such as bradycardia and hypotension, is low[21]. Recently, nifekalant has been studied as a therapy for atrial tachycardia. Di et al found that percent amplitude of fluctuation (PerAF) was efficaciously transformed to SR after a median of 7.75 minutes of pulmonary vein isolation and 30 minutes of nifekalant (50 mg) administration [22]. Morita stated that 0.3 mg/kg nifekalant had a 60-minute AFL conversion efficacy of 77.4% [23]. In our study, PSVT was successfully terminated 60% within 30 min, and the average conversion time was 27.4±10.24 min.
Nifekalant prolongs action potentials and increases the effective refractory period of atrial and ventricular myocytes [24]. Since delayed IKr in ventricular wall cardiomyocytes is heterogeneous, nifekalant may cause inconsistent ventricular wall cell repolarization and increase repolarization dispersion through the ventricular wall by prolonging the QT interval [25]. Electrocardiograms show that nifekalant prolongs the QT interval while exerting antiarrhythmic effects and lowers the defibrillation threshold, especially for various re-entry arrhythmias [26]. TdP is a severe adverse reaction to nifekalant that must be avoided by closely monitoring the electrocardiogram's QT interval. Normal QT interval prolongation peaks within 2.5 minutes of intravenous administration and fades completely 30 minutes later [27,28]. In our cases, the mean QTc interval before intravenous nifekalant administration was 412.6±17.02 ms, and the longest QTc interval after intravenous nifekalant administration was 540 ms, occurring 5 minutes after the first dose, and approximately 15 minutes after discontinuing the drug, ECG returned to normal. Non-sustained VT was seen in our study.
Study limitations
This study has several limitations. First, it was conducted at a single centre with a small number of patients. Larger, randomized, multicentre studies with longer follow-up periods are required to confirm these findings. Second, EP parameters were not systematically measured. The effects of nifekalant on various types of PVST were not investigated, so our results might not suggest the exact mechanisms of PVST termination. Third, different nifekalant doses were not used in our study. A therapeutic schedule with a higher cardioversion rate needs further exploration.
Conclusion
Nifekalant is a safe and effective option for the treatment of PVST. To evaluate the efficacy of nifekalant more precisely in PVST patients, a randomized prospective study is needed.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest influenced by the article’s content.
Funding
The project was supported by funding from the following: The project was supported by funding from the following: the National Natural Science Foundation of China [82060075]; Jiangxi Provincial Natural Science Foundation [20212BAB216057];On-campus cultivation plan of Nanchang University [PY201927]