Healy L1, Liscombe A1, Gul EE 2, Mittal A1, 3, Hong L K 1, Keelan E1, 4, Galvin J 1, 4, O’Brien J 1, Jauvert G1, Boles U 1, 5 *
1 Heart and Vascular Department, Mater Private Hospital, Dublin, Ireland
2 Department of Cardiology, Istanbul Atlas University, Medicine Hospital, Turkey
3 Cardiology Department, Southampton University Hospital, The UK
4 School of Medicine, University College Dublin, Dublin, Ireland
5 Cardiology Department, Tipperary University Hospital, Clonmel, Ireland
*Corresponding author: Usama Boles MB BCH, MSc, PhD, FRCPI, FESC, FHRS, FEHRA, Cardiologist and Electrophysiologist, Senior Lecturer Royal college of surgeons of Ireland, Heart and Vascular Center, Mater Private Hospital, Dublin & Cardiology Department, Arrhythmia Service Tipperary University Hospital, Co Tipperary, Ireland.
Received: July 22, 2023
Accepted: July 28, 2023
Published: August 08, 2023
Citation: Healy L, Liscombe A, Gul EE, Mittal A, Keelan E. (2023) “Pulsed Field Ablation for Atrial Fibrillation: A Review of Early Experience Through Clinical Cases Using a Concomitant 3-D Invasive Mapping System.” J Clinical Cardiology Interventions, 3(2); DOI: http;//doi.org/10.2023/07.1036.
Copyright: © 2023 Usama Boles. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Pulsed-field ablation (PFA) for atrial fibrillation has recently been developed which has shown clinical utility for pulmonary vein isolation ablation (PVI). In our review we discuss the mechanism of pulse field ablation, potential advantages of this technology and possible complications. However, less is known regarding the clinical benefit of 3-D concomitant mapping with PFA. Through our case series, we provide examples to reveal the superiority of the combined approach of PFA and 3 D mapping.
Methods
We reviewed 32 different studies outlining the mechanism, clinical advantages and safety of PFA from clinical development to current conventional practice and explore the benefits and potential complications. We choose and demonstrate through a series of 7 clinical cases from our institution using RHYTHMIA and CARTO 3-D mapping which to outline the clinical and practical advantages of this approach in PVI cases.
Results
While PFA technology has demonstrated clinical efficacy in patients to date. Concomitant use of 3- D mapping with PVI demonstrated advantageous abilities to achieve immediate entrance and exit block in the pulmonary veins (PVs) in all of our clinical cases. Moreover, additional use of 3D mapping to locate, identify and terminate Non PVI arrhythmias and/or delineate unusual anatomy of PVs were all revealing the superiority of such approach.
Conclusion
While PFA technology has demonstrated clinical efficacy in patients to date. Concomitant use of 3- D mapping with PVI demonstrated advantageous abilities to achieve immediate entrance and exit block in the pulmonary veins (PVs) in all of our clinical cases. Moreover, the use of 3D mapping to locate, identify and terminate non PVI arrhythmias and/or delineate unusual anatomy of PVs were all revealing the possible superiority of such approach.
I-Introduction
Radiofrequency ablation (RFA) and cryoballoon ablation (CBA) have been well established as a treatment option for refractory atrial fibrillation (AF) [1-4]. Despite technological improvement, complications such as phrenic nerve injury, atrio-oesophageal fistula and pulmonary vein stenosis seem to be intrinsically connected to invasive approaches [5]. The new non-thermal technology called pulsed-field ablation (PFA) has been recently developed, seemingly addressing many of the drawbacks associated with thermal ablation.
The focus of this review is to provide a critical evaluation to the most recent published studies on the efficacy and safety of PFA reflecting our local experience with PFA. Moreover, we present a series of cases of the additional clinical evaluation of PFA beyond PVI alone.
II-Pulsed field ablation: An overview
PFA technology works through creating high voltage electrical fields that are applied in close proximity to target tissue for a very short duration for milliseconds, to induce programmed cell death; a process called “irreversible electroporation” (IRE) [6].
Historically, the idea of electroporation has been used for several biological applications, which increased cell permeability for delivery of DNA amongst other applications. In the practice of ablation, high voltage electric fields produce short pulses to cause electroporation, resulting in tissue death without heating of the surrounding tissues, making it an appealing option for ablation [7].
Extensive preclinical studies have demonstrated the safety and efficacy of PFA using electroporation resulting in region specific destruction of myocardial tissue while avoiding damage to the surrounding tissues such as the oesophagus or phrenic nerve [7, 8-12].
Compilations of the most recent studies on the outcomes of PFA are reported in Table 1. It illustrates the effectiveness of PFA in isolating PVs, demonstrating 100% initial PVI with very few side effects, and decreased procedure times.
In a first-in-human clinical study conducted by Reddy et al. a lattice tip expandable mesh catheter was tested (Sphere 9, Inc, Watertown, MA), which could perform both RF and PFA [13]. This study achieved PV isolation in all subjects, with no observed adverse effects, demonstrating that the lattice tip catheter was a viable treatment option for AF ablation.
Moreover, Verma et al. performed a clinical study with the Medtronic Pulse select catheter where PFA was performed on 38 patients with both paroxysmal and persistent AF. 100 % PVI was achieved from first application with no associated adverse effects [14].
Biosense Webster “ Johnson and Johnson Medical, ” has been developing a system consisting of a VARIPULSE™ Catheter and TRUPULSE™ Generator [15]. This system was examined by a non-randomized inspIRE study that treated 35 paroxysmal AF patients with PFA [15].
Initial results from the study demonstrated complete PVI in all patients participating, with no associated adverse effects following the procedure, thus demonstrating the effectiveness of the IRE loop circular catheter [16]. These systems are summarized in Table 2.
III-Pulse Field Ablations advantages and safety
Thermal ablation lesion size grows exponentially with increased duration of contact, and this dependence on time critically limits attempts to improve the efficiency of thermal ablation [17]. PFA, in contrast, causes almost instantaneous lesion formation, therefore the speed of PFA means that even including the time needed for catheter manipulation and overlapping deliveries of pulses, PVI could occur in minutes. Thus, reducing total procedure time as reported [17].
A multinational retrospective study assessing clinical outcomes and efficacy by Ekanem et al. (MANIFEST-PF), involving 24 clinical centers and 1758 patients, found that most of the pericardial tamponades and strokes were related to operator manipulation and independent of energy modality [19]. Additionally, a prospective study, from Hamburg, published in 2022, demonstrated mean procedure time of 78 minutes with freedom from arrhythmia in 90% of patients with paroxysmal AF and 60% in persistent atrial fibrillation over a 12-month period.
A. Bubble formation and cerebrovascular events
Both RF and PFA have been shown to produce microbubbles in bench data and pre-clinical studies. Bubbles from RF seem to be larger, more prevalent and occur with a longer duration application compared to PFA [23]. However, no significant pathologic or embolic effects have been observed in clinical studies [21][24]. It must also be noted that readily available PFA instrumentation has the potential to introduce air, causing embolic damage, and hence frequent aspirations are strongly recommended. We trust further improvement and advances will be applied for next generations of the catheters.
B. Coronary arteries spasm and acute coronary syndrome
Coronary artery spasms have been documented during ablation over the mitral isthmus, which was noted by ST elevations in the inferior and lateral leads of the patient’s ECG following the last of 8 PFA applications in that location [25]. Intracoronary administration of nitroglycerin resolved the spasm, and the patient remained hemodynamically stable throughout the entire 16 minutes of ST elevation, waking with no chest pain or side effects [24]. A pre-clinical study performed on swine by Neven et al. used PFA to ablate along the epicardium resulting in coronary artery damage as demonstrated by acute narrowing of the coronary arteries, suggestive of coronary spasm [26]. When the coronary arteries were reassessed, they had returned to pre-ablation size, with no evidence of intimal hyperplasia on histologic examination [25].
C. PV ostial stenosis
In the PersAFOne study, PFA ablation of the PVs with biphasic, bipolar energy was investigated via cardiac computed tomography (CT) to assess for PV narrowing [22]. Baseline imagining was compared quantitatively with serial CT imaging and defined all pulmonary stenosis as >70% reduction in diameter [22]. Imaging was performed at baseline at 75 days (PEFCAT, PEFCAT II), or 90 days (IMPULSE), and all demonstrated no PV narrowing [22].
A further study investigated PV stenosis by comparing the CT images in patients acutely and 3-months post-PFA or post-RF from 4 paroxysmal atrial fibrillation trials [27]. The definition of narrowing in this study was more strict than other studies where a reduction of ostial diameter by more than 30%. At the 3-month interval, the PFA arm demonstrated 0% PV stenosis, while the RF group exhibited PV stenosis in 32.5% of patients [27].
D.Phrenic nerve palsy
The phrenic nerve is vulnerable to damage with thermal ablation due to the proximity of the nerve when ablating the right superior PV [22]. Intra procedural phrenic nerve function testing by either direct phrenic nerve pacing or by observing the diaphragm during inspiration showed no evidence of phrenic nerve palsy or paralysis following PFA [19]. Similarly, acute phrenic nerve palsy in IMPULSE, PEFCAT, and PEFCAT-II, did not demonstrate phrenic nerve palsy or paresis [21]. The effects of PFA on rat phrenic nerves also found that the location and proximity of the PFA catheter had either no effect, a modulated effect, or temporary stunning of the phrenic nerve [28]. Gross and histological investigations of phrenic nerve function following the procedure demonstrated no chronic damage [28].
The phrenic nerve is vulnerable to damage with thermal ablation due to the proximity of the nerve when ablating the right superior PV [22]. Intra procedural phrenic nerve function testing by either direct phrenic nerve pacing or by observing the diaphragm during inspiration showed no evidence of phrenic nerve palsy or paralysis following PFA [19]. Similarly, acute phrenic nerve
E.Oesophagus ulcerations
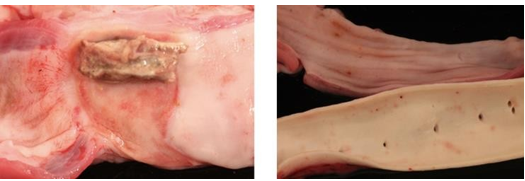
Oesophageal complications ranging from minimal mucosal ulcerations up to a rare, yet serious, atrioesophageal fistula, are not reported with PFA from 3 multi-centers with 1-year follow-up [21]. (See Figure 2.)
F.Impact on Aortic Root
PFA induced transient lesion formation on the aorta, potentially demonstrating that aortic tissue may have a lower threshold for PFA than other tissue [20]. More research must be done to effectively study this phenomenon within acute study setup.
IV- Early Local Experience of the Hybrid Approach of PFA and 3-D Voltage Guided techniques
In our institution, early experience with using PFA for PVI in conjunction to a fluoroscopy only approach, we evaluated and developed the use of a “hybrid approach” using PFA with a 3D mapping system (CARTO by Biosense Webster, Irving, United States of America or Rhythmia by Boston Scientific, Marlborough ,United States of America) using voltage guided criteria.
Components of the Combined / Hybrid Approach
The Farawave catheter is composed of 5 splines each containing 4 electrodes, which allows for manipulation of the splines from a “basket” to a “flower” configuration, with the flower form being a diameter of 31mm or 35mm [12]. Manipulation from basket to flower form allows the catheter to better adapt to individual patient anatomy when delivering the ablation energy. The catheter is housed in a 13-F (inner diameter) deflectable sheath and is inserted into the left atrium following the transseptal puncture. Ablation is delivered from each of the 4 electrodes simultaneously, while the third electrode on each spline is also able to gather local electrograms see table 3. Below.
|
Study performed |
Patient no. (N) |
PVI isolated (%) |
Procedure Complications |
Fluoroscopy time during PFA (min) |
Length of procedure PFA (min) |
|
Naktani et al., 2021 [17] |
18 |
100 |
2 groin hematomas |
23 |
96 |
|
Reddy et al., 2021 [21] |
121 |
100 |
2 pericardial effusions, 1 TIA, 1 hematoma |
13.7 ± 7.8 |
96.2 |
|
Verma et al., 2021 [14] |
38 |
100 |
1 vascular site hemorrhage |
28± 9 |
160± 91 |
|
Reddy et al. 2020 [22] |
25 |
100 |
Pericardial effusion without tamponade |
16 |
125 |
|
De Potter et al., 2021 [16] |
38 |
100 |
0 |
10.6±6.8 |
82.9±19.9 |
|
Kawamura et al., 2021 [29] |
20 |
100 |
0 |
9.1 ± 3.0 |
81.0 ± 20.0 |
|
Reddy et al., 2020 [13] |
76 (PF/PF,36; RF/PF,40) |
100 |
5 vascular complications, 2 minor esophageal erythemas (RF/PF) |
PF/PF, 2.7±2.4;
RF/PF, 5.6±4.1 |
PV isolation (PF/PF, 23.3±9.5; RF/PF, 22.0±6.8) |
Table 1.Combined data from relevant clinical studies of PFA. Procedures complications and details.
|
Company |
Catheter Style |
Waveform |
Guidance |
Reference |
|
Boston Scientific |
“Farapulse” Basket/Flower: 5 splines, 4 electrodes per spline |
Biphasic |
Fluoroscopy |
[13] |
|
Medtronic |
Circular: 9 electrodes |
Biphasic |
Fluoroscopy |
[16] |
|
Affera Inc. |
Lattice-tip catheter |
Biphasic |
Fluoroscopy |
[14] |
|
Biosense Webster |
Decapolar irrigated loop- circular catheter |
Biphasic |
Proprietary mapping system |
[15] |
Table 2. Summary of pulsed field ablation systems that are under evaluation or in use clinically.
|
CaseSeries |
|
Here we demonstrate a “hybrid” approach where a multicomponent system was utilized as follows:
All case series and images described below: |
Table 3: Describtion of the tools used for PFA in conjuction with 3D mapping.
Case 1.
A standard case using a PFA protocol devised by the advisory committee of Boston Scientific to target each PV with 8 applications (4 basket and 4 flower configurations) in paroxysmal atrial fibrillation. The hybrid approach allowed an accurate visualization of FARAPULSE electrodes and hence safer antral ablation. Validation of ablation was performed using the 3-D EA mapping using voltage criteria confirming WACA and bidirectional block.
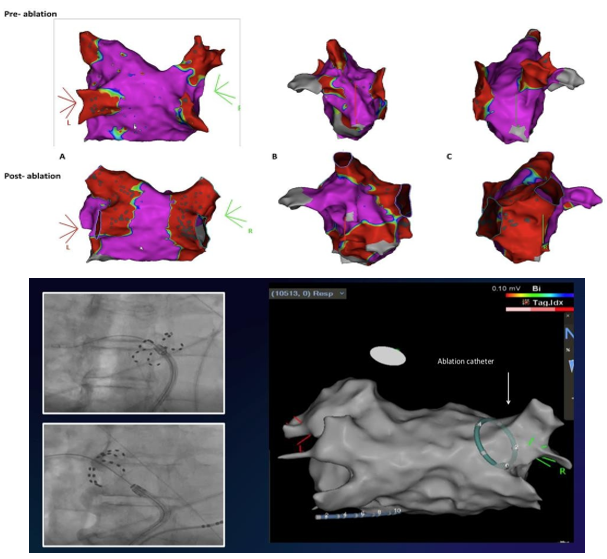
Case 1 : The 3-D electro-anatomical mapping with voltages criteria in a case of paroxysmal atrial fibrillation, comparing pre and post Bipolar Voltage after bilateral WACA A. in posterior-anterior views; B. in left lateral views and C. in right lateral views of left atrium. Lower panel shows X Ray configurations and corresponding 3 D visualization at RSPV location
Case 2.
PV and posterior wall (PW) isolation with PFA in persistent atrial fibrillation. PW isolation was achieved by four applications using a Farawave catheter in the “flower” configuration (two superior and two inferior). Validation of posterior wall isolation was determined with EA mapping.
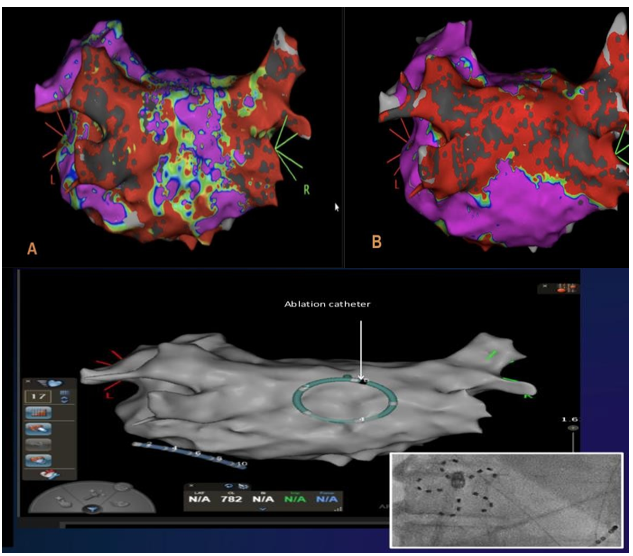
Case 2: A. LA voltage map pre PVI showing significant scarring in posterior wall of LA. B. LA voltage map demonstrating posterior wall isolation by 4 applications of PFA. Lower panel ; Farawave catheter on X ray on post. Wall clearly localized by corresponding 3D
Case 3.
PVI and PW isolation with EGMs demonstrating entrance and exit block using PFA. 3-D EA voltage mapping post ablation revealed a connected anterior RSPV that required further application of PFA in the same area. The accurate multielectrode visualization was provided by 3-D EA mapping.
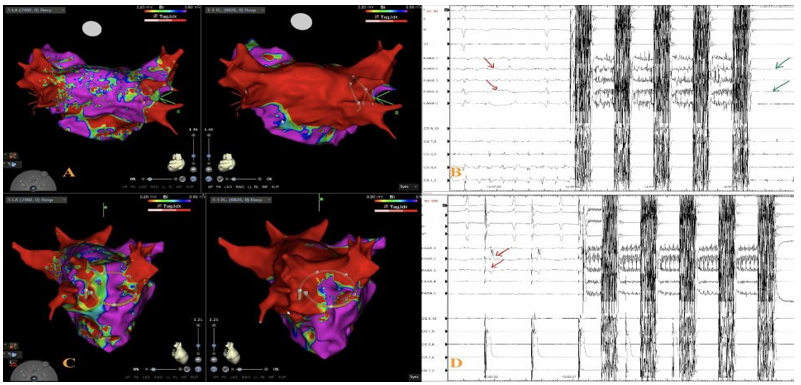
Case 3: Examples of PVs Carina with reconnections after PFA application and re- check with voltage mapping (A/C and B/D). 3D demonstrating FARAPULSE catheter position at carina requiring further applications . EGMs from Farawave catheter pre- PFA with PVPs (red arrows), after pulsed field ablation, PV isolated (Green arrows). LA Bipolar voltage map in RAO projection, pre and post ablation (C).
Case 4.
Additional use of PFA resulted in termination of peri-mitral AFL. The Local Activation Mapping (LAT) of the 3-D mapping system confirmed the mechanism of arrhythmia and gave accurate location of FARAPULSE at the peri-mitral area.
Single application resulted in normal SR reducing procedural time with entrance and exit block.
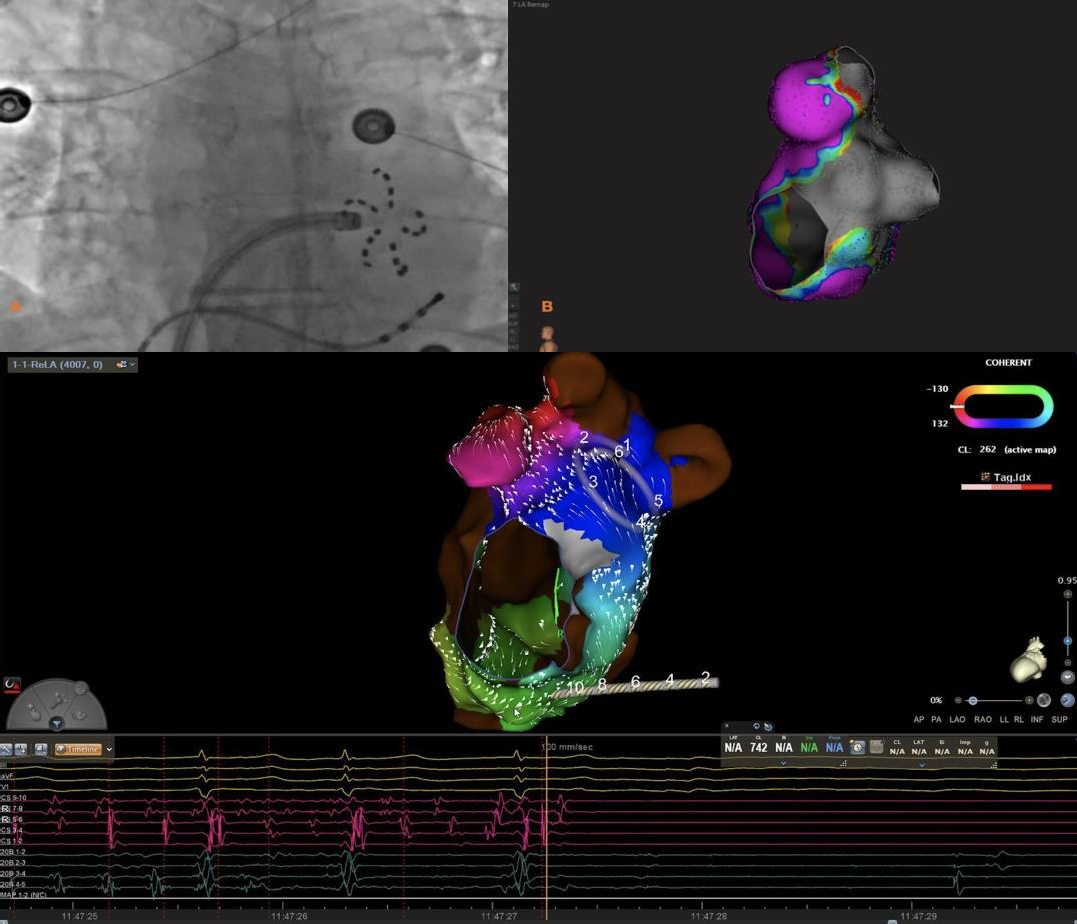
Case 4: Perimitral Atrial flutter termination using PFA. Boston Scientific RHYTHMIA. First panel left is an x-ray showing location of the catheter in mitral isthmus area in flower position. Lower panel shows LAT mapping and location of multielectrode FARAPULSE catheter. EGMs below shows termination of AFL into SR post PFA. On the right LAO view the GREY area after mitral isthmus block using voltage criteria (EAM).
Case 5.
The concomitant use of UNIVUE T.M is well appreciated to reduce x-ray dose as well as accurately assess the location of the Farapulse catheter in real time during the procedure to increased precision, without a need to increase radiation exposure.
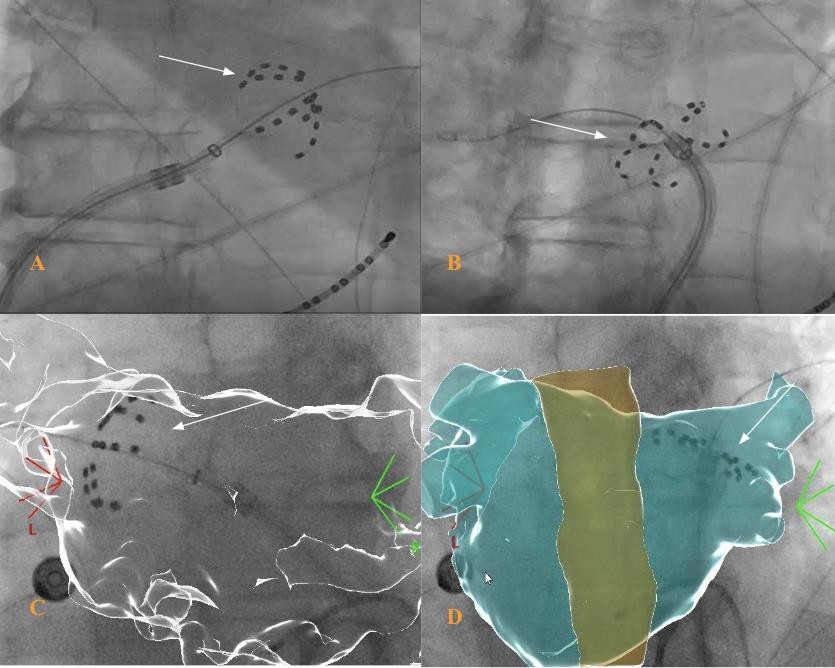
Case 5: A.Farawave catheter in basket shape in LIPV with fluoroscopy. B. Farawave catheter in flower shape in RSPV with fluoroscopy. C. CARTOUNIVU guided positioning of Farawave catheter in basket shape. D. CARTOUNIVU guided positioning of Farawave catheter in flower shape.
Case 6.
Use of PFA with 3-D mapping in a patient with a common ostium connecting left sided pulmonary veins. This demonstrates that in patients with atypical pulmonary anatomy, PFA can still provide a clinical benefit.
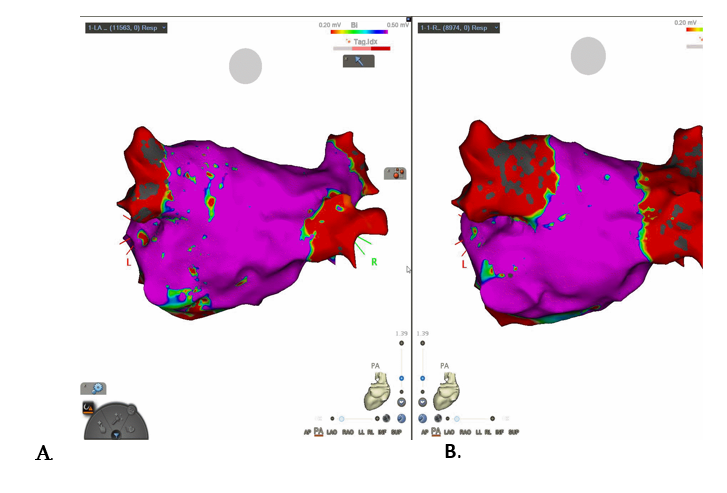
Case 6: A common left pulmonary ostium confirmed on a CT left atrium with visualisation of this on CARTO mapping pre-ablation (Image A.) and post- ablation (Image B.) This PVI was performed using PFA, FARAPULSE 31 mm catheter, with demonstration of pulmonary vein exit block on EGMs.
Use of 3-D mapping using voltages mapping confirming incomplete isolation of left PVs with an area of re-connection.
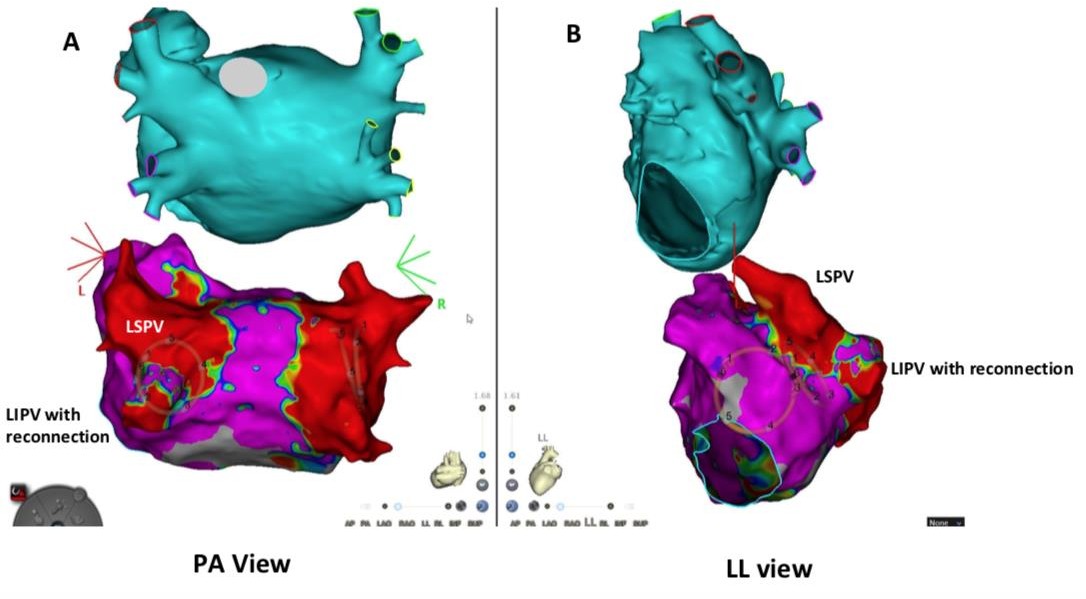
Case 7: The postero-anterior view and the left lateral view (Fig. A & B). Location of the PFA catheter can be visualized. Ablation was performed with successful exit and entrance block achieved in the left sided pulmonary veins.
Conclusion
In our case series highlighted here in table 3 at our institution, a hybrid approach demonstrated benefit in reducing fluoroscopy and radiation time, improve the mapping accuracy particularly in re-do procedures and hence ensuring superior outcomes. The hybrid approach may add more time and cost to the overall procedure, but PFA is already a consistently shorter procedure when compared with thermal ablation as demonstrated by previous studies [17-22]. The added benefit and the accuracy that the hybrid approach provides outweighs the slightly increased procedure time and costs.
Figure 1. A-B demonstrates cross-sections of right atrial appendage (RAA). C-D Demonstrates cross-section of left atrium (LA). A&C illustrate RFA lesions. B&D indicate PFA lesions. (B: arrow) PFA shows sparing of epicardial fat. RFA lesion illustrates endocardial sparing. Masson trichrome stain. Adapted from Intracardiac pulsed field ablation: Proof of feasibility in a chronic porcine model by Stewart et al.,2019, Heart Rhythm; 16:754– 764. Open access article under the CC BY-NC-ND license
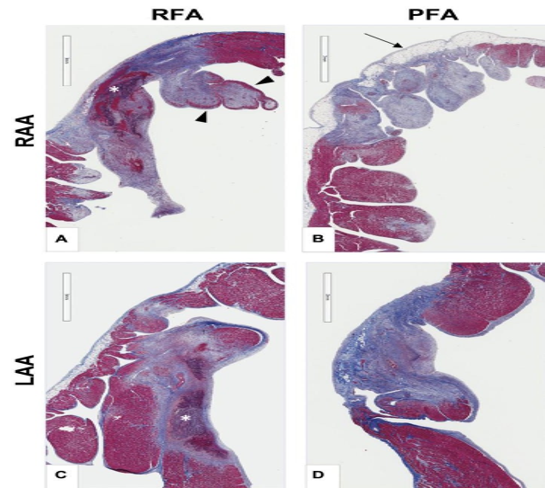
Figure 2. A Direct application of RFA on porcine esophagus, resulting in esophageal ulceration and fistula. B Direct application of PFA on porcine esophagus and aorta, with no subsequent damage. Adapted from The Promise of Pulsed Field Ablation by Haines, D, 2019, EP Digest, 19(12):1,6-10. Reprinted with permission from EP Lab Digest.