Clinical Cardiology Interventions
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2836-077X | Journal DOI: 10.61148/2836-077X/JCCI
Federico Cacciapuoti 1, Fulvio Cacciapuoti 2
1 Department of Internal Medicine “L. Vanvitelli” University of Campania – Naples, Italy
2 Department of Cardiology and C.I.C.U. ‘‘A. Cardarelli’’ Hospital – Naples, Italy
*Corresponding Author: Federico Cacciapuoti, Chair of Internal Medicine “L. Vanvitelli” University of Campania – Naples, Italy.
Received date: March 17, 2023
Accepted date: May 24, 2023
published date: July 17, 2023
Citation: Federico Cacciapuoti, Fulvio Cacciapuoti. (2023) “Insulin Resistance in Hyperhomocysteinemia Worsens Atherosclerosis.” J Clinical Cardiology Interventions, 3(1); DOI: http;//doi.org/10.2023/03.1031.
Copyright: © 2023 Federico Cacciapuoti. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Increased homocysteine levels cause the secretion of Resistin, a peptide hormone responsible for insulin resistance, that is an independent risk factor for atherosclerosis. But, insulin resistance, especially when evolves towards type 2 diabetes mellitus, favors “per se” atherosclerotic injuries regardless of diabetes.
Methods: Several mechanisms of both metabolic conditions (type 2 diabetes mellitus and hyperhomocysteinemia), in part similarly and in part differently, induce atherosclerosis. Hyperhomocysteinemia favors atherosclerosis by endothelial dysfunction and thrombogenicity, through the platelets’ activation and adhesion. Inflammatory factors and lipids’ oxidation also contribute to the atherosclerotic deposits. Likewise, the inhibition of nitric oxide synthase and the oxidative stress induces atherosclerosis. On the other hand, the impaired vasomotility and other mechanisms described for hyperhomocysteinemia further increase the vascular atherosclerosis.
Results: Our observations confirm that the coexistence of hyperhomocysteinemia and insulin resistance, especially type 2 diabetes mellitus, strengthens the incidence of atherosclerotic disease.
Conclusions: The contemporary presence of hyperhomocysteinemia and tyoe 2 diabetes mellitus often exists, even if the causes of that are unknown yet. The coexistence of these two metabolic disorders increases the cardio-vascular atherosclerotic findings.
Introduction
Some studies report that elevated levels of homocysteine (Hcy) can be associated with Insulin Resistance (IR) evolving or not toward Type 2 Diabetes Mellitus (T2DM) 1-3 and its atherosclerotic complications 4. A recent meta-analysis of Wang et al. observed that Hcy levels in patients with T2MD can be higher than in healthy individuals, especially in patients with diabetic nephropathy (DN) or diabetic retinopathy (DR) 5. Other reports have debated about a possible link between Hcy levels and insulin resistance and about the effects of folic acid and vitamin B12 supplementation on athero-vascular damage 6. Another report confirms the incidence of atherosclerotic cardiovascular disease in diabetic patients also suffering from increased Hcy (HHcy) 7. In a study performed in rodents, Li et al. found that Resistin (R), a peptide hormone secreted by adipose tissue, is involved in insulin resistance. The hormone is present in mononuclear leukocytes, in spleen, and in bone marrow cells too 8. From this last observation, a link between adipocytes (adipose cells) and diabetes seems to exist 9. On the other hand, in humans, macrophages (like adipocytes) have been found to be an important source of R 10. The relationship between HHcy and IR seems to be due to the over-expression of R from adipose tissue via endothelial reticulum stress (ERS) 11. Specifically, in mice with HHcy, this same activates C-Jun-N terminal Kinase (JNK), that inhibits the protein-kinase B(Akt) activation, participating in IR. Furthermore, JNK triggers the transcription of pro-inflammatory cytokines i, as interleuchin-6 (IL-6), interleukin-1b (IL-1b), tumor necrosis factor-a (TNF-a) and facilitating macrophage infiltration. That provokes the R expression through the activation some kinases 9,10. R incretion also causes obesity and IR considered a chronic inflammatory status 12 (Fig 1).
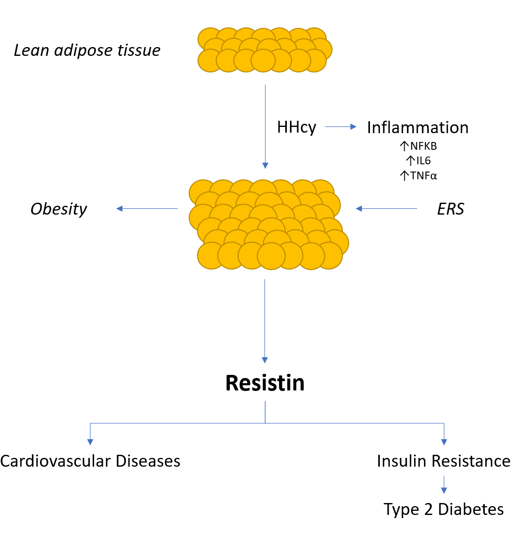
Figure 1: Resistin secreted by adipose tissue (adipocytes) induces insulin resistance and some cardiovascular diseases.
It must be added that R is associated with the development of atherosclerosis, endothelial dysfunction, cerebral and/or cardiac thrombosis, peripheral vascular diseases, inflammation and some malignancies and metastases 13-16. Therefore, a novel mechanism responsible for IR in presence of HHcy was suggested 17.
T2DM is a metabolic disease characterized by persistent hyperglycemia due to the impaired (reduced) insulin secretion and inappropriate glucagon secretion, with other possible metabolic alterations. It may cause some damages of various organs and systems, leading to several health complications. Micro-and macro-vascular diseases increase of 2-6 folds in comparison to non-diabetic population and are frequently present in diabetic patients older than 45 years 18.
Herein, we discuss about the frequency and the incidence of atherosclerotic risk derived by the contemporary presence of HHcy and T2DM.
Homocysteine
Hcy is a sulfur-containing amino acid, as intermediate product of Methionine (Met) obtainined through the re-methylation pathway 19. Another route for the metabolization of Hcy to its final products (Cysteine and Glutathione) 19 is the trans-sulfuration pathway 20 (fig.2).
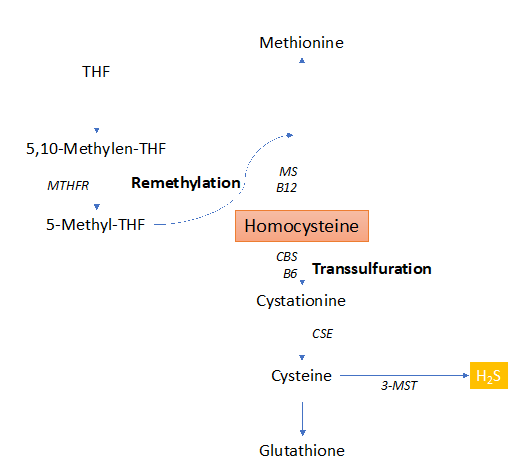
Figure 2: Homocysteine metabolism through the remethylation and trans-sulfuration pathways.
HHcy-risk factors
Some studies reported a positive correlation between HHcy and IR (with or without T2DM) 21-23. On the contrary, the Prospective Investigation of the Vasculature study in Uppsala Seniors (PIVUS) showed no evidence of plasma HHcy associated with IR 24. At present, it was demonstrated that inflammatory cytokines produced by the adipocytes through ERS, induce the over-expression of R 5. The consequent condition of pre-diabetes can evolve towards clinical T2DM. The incidence of T2DM in patients suffering of HHcy is still unknown 25. Nevertheless, some studies found that the HHcy-patients with T2DM may suffer an increased risk of atherosclerosis 25-27.
HHcy-risk factors
HHcy is recognized as a risk factor responsible for atherosclerosis 28 (Fig. 3).
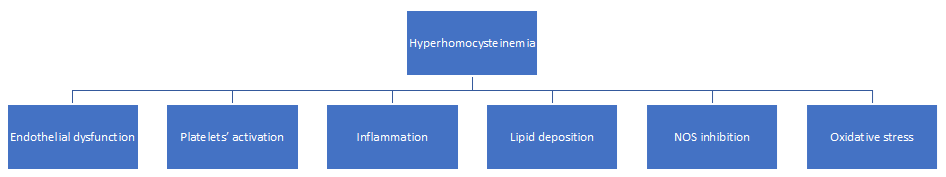
Figure 3: Mechanisms through hyperhomocysteinemia induces atherosclerosis.
It induces the endothelial dysfunction, responsible for thrombogenicity through the reduction of nitrogen monoxide (NO), a vasodilator factor produced by NO synthase 29-31. The reduction happens via asymmetric dimethylarginine (ADMA) 32. In turn, ADMA concentration rises for decreased activity of the enzyme dimethylarginine dimethyl-amino-hydrolase (DDAH) that metabolizes ADMA 33-34.
Furthermore, the alterations of the endothelial layer and the smooth muscle cells by HHcy is responsible for accelerated Reactive Oxygen Species (ROS) 35 happening for decreased expression and/or activity of key oxidant enzymes as well as the increased enzymatic generation of superoxide anion. Besides, HHcy causes the direct activation of the platelets, that increase their adhesion to the vascular endothelium. The Hcy-increased levels also favors the thrombogenicity by reactive oxygen species (ROS). This effect happens for a decreased extracellular nucleotide hydrolysis, as evidenced in rat platelets 36. On the subject, Lentz demonstrated a significant decrease in thrombomodulin anticoagulant activity 37. Signorello et al. affirmed that HHcy induces a release of arachidonic acid in the platelets, to generate thromboxane A that, in turn, activates the platelets 38. A body of evidence demonstrated that oxidative stress can be a risk factor for some important disorders, such as inflammatory diseases, including cardiovascular disease, stroke, diabetes mellitus, renal failure, and cancer 39. Moreover, activated endothelial cells have been shown to upregulate the adhesion molecules. These are responsible for monocytes and vascular cell adhesion molecule-1 (VCAM-1), release of some cytokines, chemokines, [(interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-8 (IL-8), macrophage-chemo-attractant protein-1 (MCP-1) and TNF-a)] 40,41. In addition, HHcy favors the hyperlipemia with different mechanisms, such as DNA hypomethylation 42,43, further contributing to the atherosclerotic disease 44. This association is often evident in aged patients contemporarily suffering HHcy and T2DM 45. Finally, HHcy causes the dysfunction of the matrix metalloproteinase (MMP) 46. The phenomenon happens because of a decreased elastic compliance of the vessel wall through NO isoforms 47. MMP dysfunction of the aortic wall can induce a non-frequent and dreadful clinical complication, such as abdominal aortic aneurysm, often evolving towards its rupture or inside thrombus formation 48.
IR +/-T2DM-risk factors
IR is a condition in which muscles, liver and fat don’t respond correctly to the action of insulin for the glucose use. It includes obesity (increased waist circumference), hypertension, hypercholesterolemia and T2DM (metabolic syndrome) 49. IR begins the atherosclerotic process via several mechanisms (fig.4), such as impaired vasomotility, oxidative stress, increased serum levels of very low-density lipoproteins (VLDLs), triglycerides, and low-density LDLs-cholesterol 50.
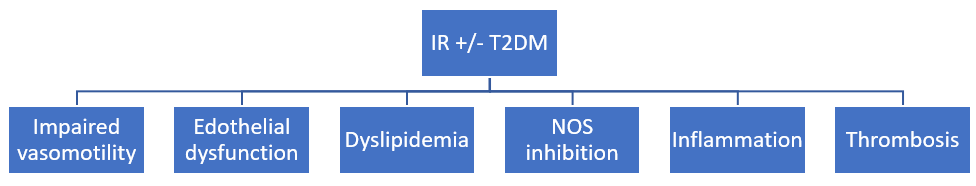
Figure 4: Mechanisms through the insulin resistance (IR)/type 2 diabetes mellitus (T2DM) causes atherosclerosis.
Dense LDL present in patients with IR and/or T2DM are strongly predictive of atherosclerotic events. These enter the arterial wall, causing the toxic effect of endothelial cells. In addition, the oxidation of LDL lipoproteins by ROS formation and reactive nitrogen species (RNS), plays a key role in the initiation and development of atherosclerosis. That induces an endothelial dysfunction and vascular smooth muscle cells (VSMCs) proliferation, while LDL accumulation leads to foam cells formation, further favoring the atherosclerotic lesions 51,52. Referring to the vascular endothelium, it is known that this is an endocrine organ involved in the regulation of vascular tone. It plays a fundamental role in the maintenance of vascular homeostasis. The function depends on production of some mediators able to regulate vascular tone. The balance among endothelium-derived vasodilative substances (NO, prostaglandins, derived hyperpolarization factor, etc.) and vasoconstrictors (Angiotensin II, prostanoids, isoprostanes) is responsible for the normal vascular contractility, while the prevalence of vasodilative factors on vasoconstrictors or vice versa causes an impaired vascular tone. The vasodilator factors have anti-proliferative and anti-inflammatory effects, while the vasoconstrictors are mitogenic and favor the inflammation. Mitogen activity consists in the proliferation of VSMCs, responsible of vascular wall’s fibrosis, while the inflammatory activity causes the production of pro-inflammatory cytokines, growth factor, interleukines and others. Particularly, cytokines are associated with vascular dysfunction and atherosclerosis, abdominal aortic aneurysm, and systemic hypertension 51. These activities contrast with normal endothelial function, reduce the expression of vascular cell adhesion molecules, attenuate the production of pro-inflammatory cytokines, decrease leukocytes recruitment, inhibit VSMC proliferation, attenuate platelet’s aggregation and reduce monocytes adhesion 53-57. Therefore, while the normal endothelial function depends on insulin sensitivity, endothelial dysfunction is strongly favored by IR 58,59.
Finally, in these patients, metabolic disorder disturbs the physiological balance of coagulation and fibrinolysis. Specifically, hyperglycemia and IR upregulate level of the pro-coagulation mediators, like tissue factor, thrombin, and some coagulative factors such as FVII, FXI, FXII, etc. On the other hand, diabetes contributes to cardiovascular changes and reduces fibrinolysis by decreasing tPA and increasing PA-I, contributing to generate clots 60-63. Other factors frequently present in diabetic patients, such as obesity and dyslipidemia, also contribute to coagulation disorders and are prone to thrombus generation 63.
Conclusive remarks
Conclusively, the coexistence of HHcy and IR with or without T2DM and obesity, significantly increases atherosclerotic changes through several mechanisms 4-64. Among possible mechanisms of increased atherosclerotic findings rising from the coexistence of two defective metabolic syndromes are included the inflammatory status and the increased procoagulant activity, especially in diabetic nephropathy 65,66 and endothelial dysfunction 67. It must be also added that cardiovascular changes happening in these patients aren’t just the number of atherosclerotic marks induced by HHcy and IR separately esteemed, but a strengthened result derived by the contemporary presence of two metabolic disorders. But the modalities through both HHcy-factors and IR-factors contemporarily act in induce and accentuate atherosclerotic cardio-vascular derangement act are still unknown.
Conflict of Interest: The authors declare they have no conflict of interest.