Clinical Cardiology Interventions
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2836-077X | Journal DOI: 10.61148/2836-077X/JCCI
Sergey Belyakin1,*, Sergey Shuteev 1,2
1Department of General Physics, Physics Faculty, Lomonosov Moscow State University, Moscow, Russia.
2Laboratory of dynamic systems, Physics Faculty, Lomonosov Moscow State University, Moscow, Russia.
*Corresponding author: Sergey Belyakin, Department of General Physics, Physics Faculty, Lomonosov Moscow State University, Moscow, Russia.
*Corresponding author: Sergey Belyakin, Department of General Physics, Physics Faculty, Lomonosov Moscow State University, Moscow, Russia.
Received: February 06, 2021
Accepted: February 18, 2021
Published: February 24, 2021
Citation: Belyakin S, Shuteev S. (2021) Impact of Inflammation on Atrial Fibrillation in Patients with Metabolic Syndrome. Cardiology Interventions, 2(1); DOI: http;//doi.org/03.2021/1.1006
Copyright: ©2021 Sergey Belyakin, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1. Introduction
In a healthy heart, refractoriness provides a normal sequence of propagation of excitation into the heart and electrical stability of the myocardium. Since the area of the myocardium through which the excitation passes becomes unresponsive for some time, re-entry of the excitation into this area is impossible. Due to this, the counter waves in the myocardium mutually "extinguish" each other, which prevents, in particular, the appearance of unwanted circulation of excitation. However, in the final stage of each excitation cycle, the myocardium becomes inhomogeneous in refractoriness for a short time and loses electrical stability. Stimulus, acting at this time, can lead to serious violations of the normal course of excitation, in particular to the emergence of circulating excitation waves by the mechanism of "re-entry" (re-entry) [3,4].
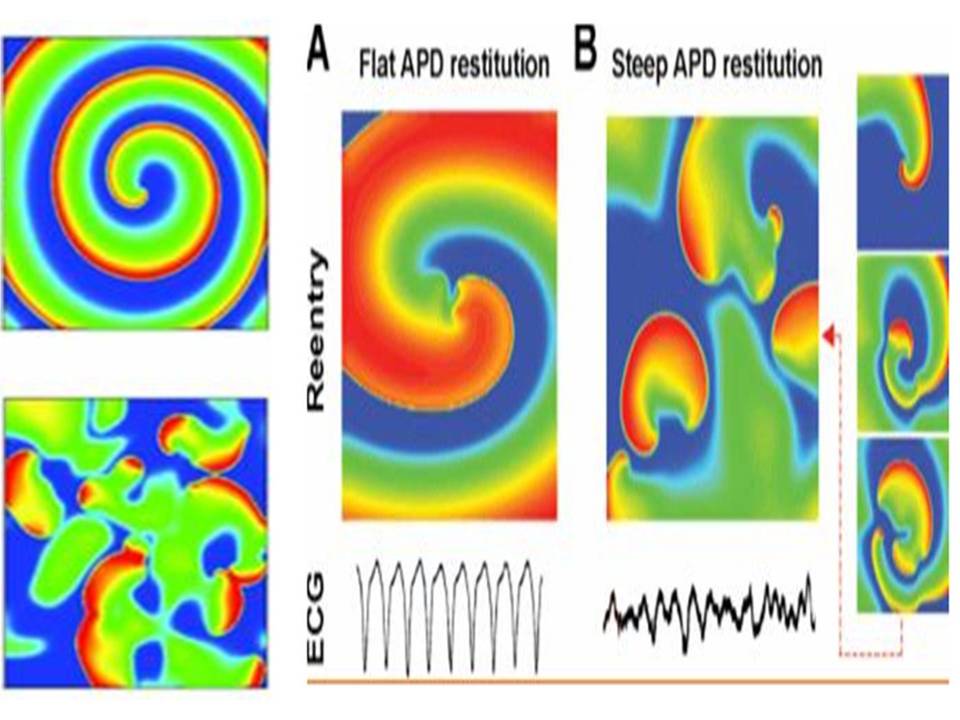
Figure 1: Stable spiral wave when the APD recovery curve is flat. Electrical recovery and stability of a spiral wave in a homogeneous two-dimensional tissue. A – the restoration curve of APD is flat, induced reentry (spiral waves) is stable and corresponds to monomorphic tachycardia. B – recovery curve APD steep (steep).
Sharp violations of the normal ratio of excitability and refractoriness can lead to the formation of a large number of re-entrant waves in the myocardium, which are spiral waves (Fig.1) on the surface of the heart and complete desynchronization and discoordination of the activity of the myocardial fibers when they begin to excite and contract independently. This condition is called fibrillation and is accompanied by almost complete loss of pumping function of the corresponding part of the heart. Ventricular fibrillation (VF) is an extreme form of cardiac arrhythmias and the predominant cause of sudden death (due to circulatory arrest) among patients with CVD. Therefore, this pathology is studied particularly intensively. However, there is still no consensus on the mechanism supporting the FF. In one case, it is believed that the LF is due to a single rapidly rotating wave, or the mother rotor, which can be both stationary and wandering, and in interaction with the inhomogeneities of the heart tissue can initiate secondary turbulent activity [5]. In another case, it is assumed that a complex pattern of excitation during LF is formed by multiple re-entrant waves. These two options do not exclude each other, and recent studies have shown that in isolated rabbit hearts, both types of LF can exist [5]. In the dissertation work we adhere to the widespread hypothesis about the presence of multiple rotors during fibrillation in large hearts [6], for example, a person may have about ~ 15 of them. The theory of dynamic systems describes many processes inherent in active media, including some types of arrhythmias [7]. Since arrhythmias are caused by certain disorders in the heart muscle and, therefore, are pathological conditions, the modeling of such systems is of great practical interest and can bring closer to solving the problem of the possibility of controlling their behavior through external influences. This, in turn, allows us to come close to the problem of soft withdrawal of active systems from the state of developed space–time chaos that characterizes some types of pathologies [8,9].
2. The mathematical dynamic model of the soliton
The mathematical dynamic model of the soliton is represented by the equation (1).
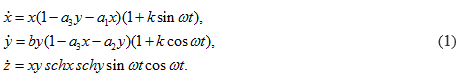
Time portraits of the system (1) are shown in Fig.(2–4), active relaxation media:
a =1.0, a1 = 0.2, a2 = 0.1, a3 = 1.0, b = –2.0, k = 0.045, ω = 64π.

Figure 2: Time portraits of the system (1) at : x0 = 0.4, y0 = 0.4, z0 = 2.5, t = (0→ 25).
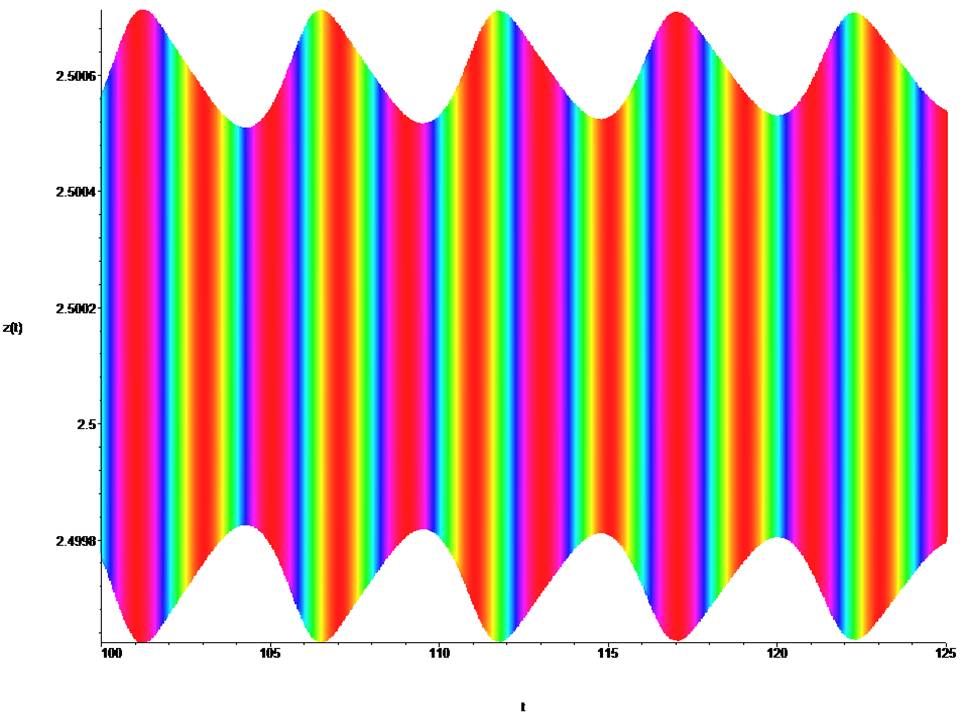
Figure 3: Time portraits of the system (1) at : x0 = 0.4, y0 = 0.4, z0 = 2.5, t = (100→ 125).
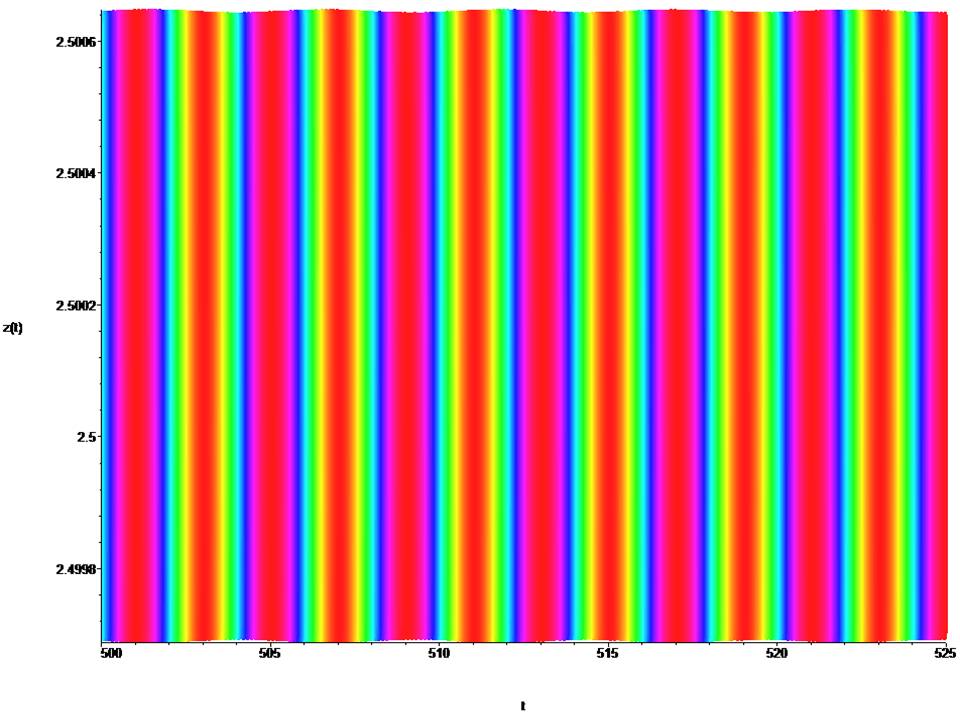
Figure 4: Time portraits of the system (1) at : x0 = 0.4, y0 = 0.4, z0 = 2.5, t = (500→ 525).
Where: z – the classical soliton equation, (x,y) – determine the chirality of the system, (a,b) – initial amplitudes of stimulation, k – minimum internal potential, a1,a2,a3 – internal state (dispersion), ω – angular spiral frequency.
3. The classical soliton model of the normal state of the heart rhythm in the absence of fibrillation
Time portraits of the system (1) are shown in Fig.(5), active relaxation media:
a =±1.0, a1 = 0.2, a2 = 0.1, a3 = 1.0, b =±2.0, k = 0.045, ω = 64π.

Figure 5: Time portraits of the system (1) at : x0 = 0.4, y0 = 0.4, z0 = 2.5, t = (–3→ 3).
4. Classical soliton model heart failure rhythm presence of fibrillation
Time portraits of the system (1) are shown in Fig.(6), passive dispersed media:
a =±0.09, a1 = 0.2, a2 = 0.1, a3 = 1.0, b =±0.09, k = 0.045, ω = 64π.

Figure 6: Time portraits of the system (1) at: x0 = 0.09, y0 = 0.09, z0 = 0.09, t = (–70→ 70).
5. The classical model of the soliton is heart failure of the rhythm, the presence of fibrillation under external force
Time portraits of the system (1) are shown in Fig.(7,8), passive dispersed media under external force: a =±0.09, a1 = 0.2, a2 = 0.1, a3 = 1.0, b =±0.09, k = 0.045, ω = 64π.
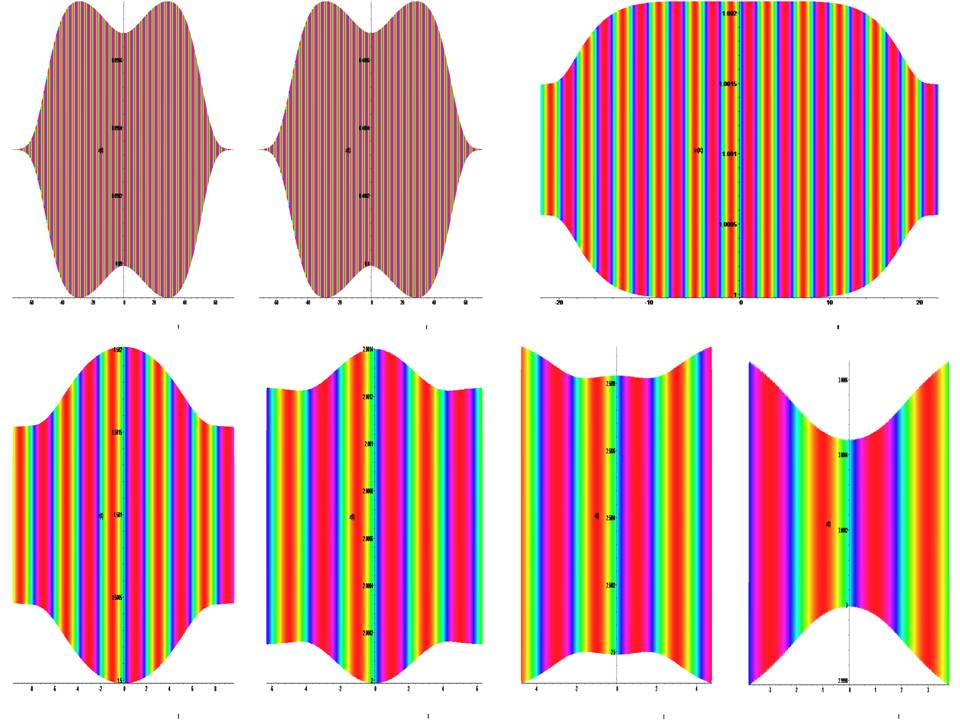
Figure 7: Time portraits of the system (1) at: x0 = y0 = z0 → (0.09, 0.4, 1.0, 1.5, 2.0, 2.5, 3.0).

Figure 8: Time portraits of the system (1) at: x0 = y0 = z0 → (3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0).
4. Result
The mathematical dynamic model of the soliton is represented by equation (1) for a =1.0, a1 = 0.2, a2 = 0.1, a3 = 1.0, b = –2.0, k = 0.045, ω = 64π and describes the active relaxation state. At t = (0→ 250), a cylindrical shock wave forms the leading edge of the wave (fig. 2,3). At t = (300→ 500), the cylindrical shock wave is formed into a classical frontal wave (fig. 4). The mathematical dynamic model of the soliton is represented by equation (1) for a = ±1.0, a1 = 0.2, a2 = 0.1, a3 = 1.0, b = ±2.0, k = 0.045, ω = 64π and active relaxation media. At t = (–3→ 3), a cylindrical shock wave forms the back front of the wave (fig. 5). The mathematical dynamic model of the soliton is represented by equation (1) for a = ±0.09, a1 = 0.2, a2 = 0.1, a3 = 1.0, b = ±0.09, k = 0.045, ω = 64π and passive dispersed media. At t = (–70→ 70), a this condition corresponds to fibrillation and is shown in the (fig. 6). The fig. (7,8) show the suppression of fibrillation. When this condition occurs (x0 = y0 = z0 = 1.0 ), the stabilization of the rhythm. Under this condition (x0 = y0 = z0 = 1.5 ), a soliton is formed that stabilizes the rhythm. Under this condition (x0 = y0 = z0 = 3.0 ), bifurcation and destabilization of the rhythm occurs. Under this condition (x0 = y0 = z0 = 7.0 ), instability and destruction of the rhythm occurs. From this it follows that to suppress fibrillation, you can not use a defibrillator, since its force effect is in the hundreds, and a stun gun in 1000, which is categorically unacceptable.
Summary:
A modified nonlinear fibrillation model based on the classical model of a twisting spiral and two coupled classical solitons is developed. .This system of equations (1) can be useful for studying and predicting the behavior of processes in a homogeneous medium, dispersion, and active medium (human atrial tissue) [10-18].