Clinical Cardiology Interventions
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2836-077X | Journal DOI: 10.61148/2836-077X/JCCI
Ylber Jani M1, Kastriot Haxhirexha2, Nehat Baftiu 3*, Bekim Pocesta MD4, Atila Rexhepi 5 , Sotiraq Xhunga 6, Artur Serani 7, Fatmir Ferati 8, Dali Lala MD9, Agim Zeqiri MD 10, Ahmet Kamberi 11
1,2,5,8 Faculty of Medicine,Tetovo Republic of North Macedonia
3 Faculty of Medicine QKKUK Pri shtina Republik of Kosovo
4 Department of Cardiology Faculty of Medicine"Ss Kiril and Metodij" University Skopje Republic of North Macedoni 6,7 Department of Cardiology Medical Center Dures Republic of Albania 9 Private Health Institute of family medicine "Florenc "Tetovo Republic of Macedonija 11 Department of Cardiology Faculty of Medicine M.Teresa Tirana Republic of Albania.
*Corresponding author: Nehat Baftiu, Faculty of Medicine QKKUK Pri shtina Republik of Kosovo
*Corresponding author: Nehat Baftiu, Faculty of Medicine QKKUK Pri shtina Republik of Kosovo
Received: January 12, 2021
Accepted: January 20, 2021
Published: February 16, 2021
Citation: Ylber Jani M, Kastriot Haxhirexha, Nehat Baftiu, Bekim Pocesta Atila Rexhepi, Sotiraq Xhunga, Artur Serani, Fatmir Ferati, Dali Lala, Agim Zeqiri, Ahmet Kamberi, (2021) Impact of Inflammation on Atrial Fibrillation in Patients with Metabolic Syndrome.. Cardiology Interventions, 2(1); DOI: http;//doi.org/03.2021/1.1005
Copyright: ©2021 Nehat Baftiu, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Inflammation have been involved in the pathogenesis of both metabolic syndrome(MS) and atrial fibrillation(AF). The magnitude of elevations in plasma C-reactive protein(CRP) a marker of inflammation, is probably related to atrial structural remodeling and impaired atrial function.In patient with MS,limited data exist regarding impact of plasma levels of inflammatory markers,such as C-reactive protein on the: type of AF and atrial structural and functional remodeling.
Objective: We set to analyze the impact of degree of systemic inflammation (assessed acrording to CRP levels),on the type of AF,atrial functional and structural remodeling in patients with MS.
Methods: We conducted an multicenter observational cross-sectional study. Recruited were 425 consecutive participants,with MS and AF(paroxysmal, persistent and permanent AF),who attended outpatient visits at 7 general cardiology Health Care Clinics, during 1 calendar year,stratified according CRP-levels:(211 participans with level of CRP ≥3mg/l, and 214, with level of CRP <3mg/l).
Results: Permanent type of AF, was more common in participans with CRP-levels ≥3mg/l(54.6% vs.20.5%,p= 0.000),whereas parhoxysmal AF, was more common in participans with CRP-levels of <3 mg/l (9.9% vs.52.3%,p=0.000).
Patients who had CRP levels above the cut-off of 3 mg/l,had increased dimension of left atrium{(LA),(4.2±0.3 vs. 3.7±0.2,p=0.000)},higher prevalence of enlargement of LA {defined as left atrial volum index(LAVI) ≥29ml/m2(84.1% vs.48.5%,p=0.002)} and inverse relationship of LA function(defined as left atrial emptying fraction(LAEF <45%, (30.8±3.4 vs.41.9±2.6, p=0.00).
There was observed significant association of CRP levels above the cut-off of 3 mg/l and: frequence of persistent AF(OR=8.824,95% CI 1.689-46.100), permanent AF(OR=13.955, 95%CI 2.676 -72.780),increased LA dimension (OR=3.817,95% CI 0.989 -1.544), and decreased LA function.{(expressed by: LAVI >29ml/m2OR=4.014, 95% CI 2.620-6.152),LAEF <45%(OR=3.323,95%CI 2.062 -5.351) and LAVI>29 + LAEF-reduced (OR=3.354,95% CI 1.693 - 6.646)}.
Conclusions: We proved the hypothesis that different degree of inflammation have variable inpact on the different types and duration of Atrial fibrillation,and variable impact on the atrial functional and structural remodeling, in the patient with MS.
Introduction:
Metabolic Syndrome(MS) and Atrial fibrillation(AF) are common disorders,ass-ociated with increased cardiovascular desease morbidity and mortality.Their prevalence is constantly increasing with the growth of the elderly population and changing lifestyle,becoming major health problem [1,2]. Metabolic syndrome represents a cluster of atherogenic risk factors, all of these risk factors could influence the development of atrial fibrillation, an association between atrial fibrillation and the metabolic syndrome has been suggested [3].Several studies reported that MS is associated with atrial electrical and structural remodeling4,but the mechanisms that relate metabolic syndrome to the increased risk of atrial fibrillation occurrence, are not completely understood [2,5], this requires a better understanding of the mechanisms underlying AF, with the premise that improved mechanistic insights will lead to innovative and more effective therapeutic approaches.
Experimental and clinical data indicate that inflammation have been involved in the pathogenesis of both metabolic syndrome and atrial fibrillation. Considering that inflammation could induce atrial electrical and structural remodeling, it is reasonable to assume that inflammation might also facilitate the development of AF in the context of MS [6].
A marker of systemic inflammation, such as C-reactive protein (CRP),rise in patients with AF [7]. The magnitude of elevations in plasma CRP is probably related to atrial structural remodeling and impaired atrial function [8,9]. In patient with MS,limited data exist regarding impact of plasma levels of inflammatory markers,such as C-reactive protein on the type of AF,atrial structural and functional remodeling [10,13].We tested the hypothesis:The different levels of C-reactive protein have different inpact on the type of AF,degree of atrial structural and functional remodeling in the patient with MS.These findings might lend further insight into impact of inflammation on clinical presentation of AF,atrial structural remodeling and atrial function in patients with MS.
Objective: We set to analyze the impact of degree of systemic inflammation assessed acrording to CRP levels, on the type of AF,atrial functional and structural remodeling in patients with MS.
Methods:
Study design
We conducted an multicenter observational cross-sectional study.Recruited were 425 consecutive participants,with MS and Atrial Fibrillation{(AF),(paroxysmal, persistent and permanent AF)}, defined on the basis of the classification guidelines of ESC [14],who attended outpatient visits at 7 general cardiology Health Care Clinics, during 1 calendar year (from May 2019 to June 2020),stratified in two group:211 participans(100 females and 111 males) with level of CRP ≥3mg/l, and 214(96 females and 118 males) with level of CRP <3mg/l.
Health screening included a physical examination,resting ECG [14], anthropometrics, blood pressure(BP) obtained after 10 min of rest in the sitting position, expressed as the average of 3 consecutive measurements. Hypertension was defined as a systolic blood pressure ≥140 mmHg,diastolic blood pressure ≥90 mmHg and/or current anti-hypertensive therapy [15].Diabetes mellitus was defined as a fasting serum glucose level ≥126 mg/dL and/or current medical therapy with an oral hypoglycemic agent and/or insulin [16].
Weight was obtained with an electronic scale, rounding the number to nearest 0.1 kg. Height was measured without shoes, rounding the number to nearest 0.5 cm. Body mas index(BMI) was calculated as weight (kg), divided by the height (m) squared [17]. Waist circumference (WC) was measured after a normal expiration with patient standing up, wearing only underwear, rounding the number to nearest 0.5 cm. The measure was taken in the mid-point between the bottom of the lowest rib and the top of the iliac crest [18]. WC,was reported in cm.An overnight fasting blood sample, was drawn from each patient to determine: blood glucose, lipid profile tests total serum cholesterol(TC), serum High density lipoproteins cholesterol (HDL-C), serum triglycerides (TG).The sample analysis was performed using standard biochemical analytical methods.
Plasma CRP levels was measured using latex particle-enhanced immunoassay with the mephelometry(Roche Swiss).Consistent with recommendations from Centers for Disease Control and Prevention [19],a (CRP cut point of 3.0 mg/l),was used to differentiate high-risk and low-risk group.
Metabolic Syndrome, was defined according to the harmonized definition of the International Diabetes Federation and other organizations [20].On the basis of the baseline examination, the metabolic syndrome was diagnosed when at least 3 of the following criteria were met.(1) central adiposity {Waist circumference (WC)} >102 cm in men and >88cm in women); (2) serum HDL-C < 50 mg/dL in women or < 40 mg/dL in men; (3) serum triglyceride levels > 150 mg/dL; (4) SBP ≥ 130mm Hg or DBP ≥ 85mm Hg or use of antihypertensive drugs;(5) the presence of diabetes mellitus(DM) or use of anti-diabetic drugs.
Conventional Echocardiography:
M-mode, two-dimensional and Doppler echocardiography, were performed and/or reviewed by experienced staff cardiologists, compliant with the recommendation of the American Society of Echocardiography and the European Association of Cardiovascular Imaging [21], stored in DICOM format and later reviewed by two experienced echocardiographers. Briefly, left ventricular (LV) volumes and ejection fraction (LVEF) were calculated by the modified Simpson's method. The left ventricular mass was calculated from LV linear dimensions using the ASE recommend formula (0.8 × {1.04[(LVIDd + PWTd + SWTd)3 − (LVIDd)3]} + 0.6 g) and indexed to the body surface area, with LV hypertrophy (LVH) defined as LV mass index (LVMI) >115 g/m2 in men or >95 g/m2 in women [22].
Assessment of LA Volumes and LA phasic functions:Left atrial(LA) diameter was the 2D anterior–posterior length in the parasternal long-axis view. We defined LA size: as normal (< 2.2 cm/m2), moderately enlarged (2.2–2.79 cm/m2) and severely enlarged (≥ 2.8 cm/m2) [23].LA phasic function we derived from following volumetric measurements:Left atrial maximum volume(LA max vol), was measured by the modified Simpson's method using apical four- and two-chamber views at the end-systolic frame preceding mitral valve opening, and was indexed to the body surface area to derive the LA volume index (LAVI). Elevated LAVI was definite as (LAVI ≥ 29 mL/m2).Similarly, LA minimum volume(LA min vol) was measured at the end-diastolic frame preceding mitral valve closure, left atrial emptying fraction (LAEF) was calculated using recommended formula (EF= LA max vol – LA min vol)/LA max vol). Reduced LAEF was defined as ≤45% [24,25].
Final values were taken as the mean of measurements from three cardiac cycles. Atrial fibrillation at the time of echocardiography was determined by the absence of A-waves on transmitral spectral Doppler flow as well as the lack of organized electrocardiographic P-waves.
Patients with a history of coronary artery disease, left ventricular(LV) wall motion abnormality, and ejection fraction of <50%, valvular heart disease, primary cardio myopathy, bundle branch block,anemia,electrolyte imbalance, renal failure,pulmo-nary disease, subjects with more than mild valvular regurgitation (assessed Quali-tatively with color Doppler imaging) and valvular stenosis of any extent were also excluded, patients with poor quality echocardiographic and electrocardiographic images also were excluded.
All patient were written informed, consent was obtained from all participating patients before they were enrolled into the study.
Statistical Analysis.
For evaluation of the data obtained from the study, descriptive statistical methods of mean ± standard deviation, frequency and ratio values were used.The distribution of variables was tested for normality using the Kolmogorov-Smirnov test, and the heterogeneity of variances was evaluated by Levene's test.Analysis of quantitative variables with normal distribution was performed using the Student- t test and analysis of variance (ANOVA).Continuous variables with abnormal distribution and qualitative variables were analyzed using the χ² test.The association between variables were analyzed using logistic regression,odds ration (OR) and 95% confidence interval(CI) were estimated.A,p value <0.05,was considered statistically significant for a confidence interval of 95%.
Statistical analyses were performed with the SPSS software package (SPSS 19.0).
Results:
From September 2019, through September 2020, we enrolled 425 consecutive participants,with MS and Atrial Fibrillation in the study. The median follow-up duration was 1.0 years .
|
Variables |
MS (N.450) |
P - value |
||||||
|
Gr. with CRP levels ≥ 3mg/l (n-211) |
Gr.with CRP levels < 3mg/l (n.214) |
|||||||
|
N. (%) |
Mean |
±SD |
N. (%) |
Mean |
±SD |
|||
|
Gender,Female |
94(44.6) |
|
|
100(47.4) |
|
|
0.7 |
|
|
Age (year) |
|
61.5 |
±2.7 |
|
60.2 |
±3.4 |
0.8 |
|
|
BMI(kg/m2) |
|
27.8 |
±4.1 |
|
26.6 |
±3.0 |
0.04* |
|
|
AF(paroxysmal) |
21 (9.9) |
|
|
112 (52.3) |
|
|
0.000* |
|
|
AF(persistens) |
75 (35.5) |
|
|
58 (37.1) |
|
|
0.1 |
|
|
AP(permanent) |
115 (54.6) |
|
|
44 (20.5) |
|
|
0.000* |
|
|
H.T.A (presence) |
157 (74.5) |
|
|
168 (78.5) |
|
|
0.7 |
|
|
H.T.A (uncontrolled) |
88 (41.7) |
|
|
129 (60.3) |
|
|
0.03 |
|
|
T2DM (presence) |
164 (77.6) |
|
|
160 (74.7) |
|
|
0.8 |
|
|
WCi (presence) |
175 (82.7) |
|
|
179 (83.6) |
|
|
0.9 |
|
|
HDL-chl(presence) |
137 (65) |
|
|
134 (62.6) |
|
|
0.7 |
|
|
TG (presence). |
118 (55.9) |
|
|
123 (57) |
|
|
0.8 |
|
|
CRP(mg/dL) |
|
6.8 |
±3.6 |
|
2.7 |
±0.2 |
0.000* |
|
|
Three MS risk fac. |
137 (65) |
|
|
87 (40.6) |
|
|
0.005 |
|
|
Four risk fac.MS |
52 (24.6) |
|
|
86(40.1) |
|
|
0.01 |
|
|
Five risk fac.MS |
22 (10.4) |
|
|
41 (19.1) |
|
|
0.02 |
|
AF,HTA,T2DM,WCi,HDL-chol;TG,Three,Four,Five{(risk factors for MS)-presence.(presence among patients in groups)}.
Table 1: Baseline characteristics of patients with MS (n.425),according to CRP levels.
Table 1 shows baseline characteristics of patients by CRP levels. Overall,participans with CRP-levels of ≥ 3mg/l,have higher:body-mass index,more risk factors for MS and higher BP.( 27.8 ±4. vs. 26.6±3.0,p=0.04; four risk factors for MS 40,1% vs. 24.6 %,p=0.001;five risk factors for MS 19.1% vs. 10.4%, p=0.02; uncontrolled BP 60.3% vs. 41.7%, p=0.03).
Permanent type of AF, was more common in participans with CRP-levels ≥3mg/l(54.6% vs.20.5%,p= 0.000),whereas parhoxysmal AF, was more common in participans with CRP-levels of <3 mg/l (9.9% vs.52.3%,p=0.000).Ther was not significant changes between groups in relation to frequency of persistent AF.(Figure.1).
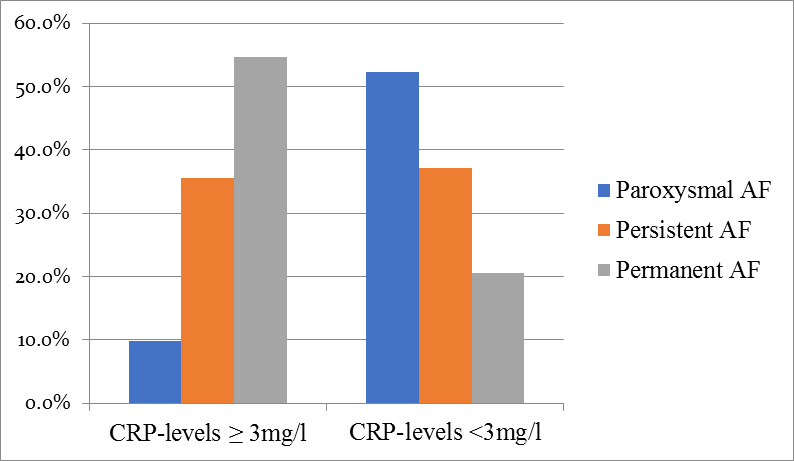
Figure 1: Frequency of Atrial Fibrilation according to CRP-lvels.Duration of Atrial Fibrilation significantly increase among those with higher CRP-levels.
Echocardiographic data of cardiac structure and function according to CRP levels, are presented in Tables 2 and 2A.
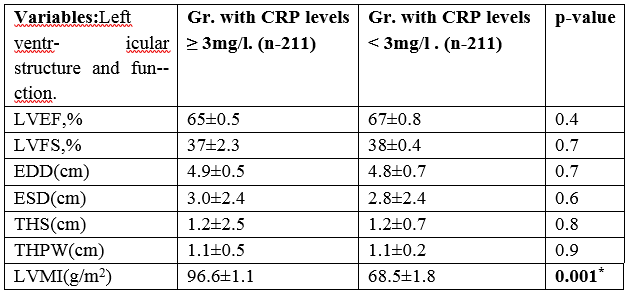
Table 2A: Echocardiografic data of cardiac structure and fungtion according to CRP levels.
There was not significant changes between groups in relation to left ventricular dimensions and ejection fraction, but in relation to LVMI there was significant difference. Patients who had CRP levels above the cut-off of 3 mg/l, had higher LVMI than patients with levels below 3 mg/l respectively (96.6±1.1 vs. 68.5±1.8,p=0.001).(Table.2).
|
Variables:Left Atrial structure and function. |
Gr. with CRP levels ≥ 3mg/l. (n-211) |
Gr. with CRP levels < 3mg/l . (n-211) |
p-value |
|
LA.dimension (cm). |
4.2±0.3 |
3.7±0.2 |
0.000* |
|
LAVI.max.vol.(ml/m2) |
39,6±2.9 |
31.7±1.6 |
0.000* |
|
LA.min vol.(ml) |
44.8±6.2 |
36.1±3.6 |
0.000* |
|
LA.emtying fraction(%) |
30.8±3.4 |
41.9±2.6 |
0.000* |
|
LAVI>29 ml/m2;(n;%) |
177 (84.1) |
104 (48.5) |
0.002* |
|
LAEF < 45% (n;%) |
181 (85.7) |
138 (64.4) |
0.001* |
|
LAVI>29 +LAEF-r (n;%) |
157 (74.4) |
94 (43.9) |
0.001* |
|
LAVI-n +LAEF-n (n;%) |
8 (3.8) |
37 (17.2) |
0.001* |
|
LAVI-n +LAEF-r (n;%) |
30 (14.2) |
73 (34.1) |
0.002* |
|
LAVI>29 +LAEF-n (n;%) |
10 (4.7) |
16 (7.4) |
0.2 |
LAVI-n; Normal left atrial volum index;LAEF-r; Reduced left atrial emptying fraction.LAEF-n;Normal left atrial emptying fraction.
Table 2B: Echocardiografic data of cardiac structure and fungtion according to CRP levels.
There was significant changes between groups in relation to dimension and function of LA.(Fig.2).
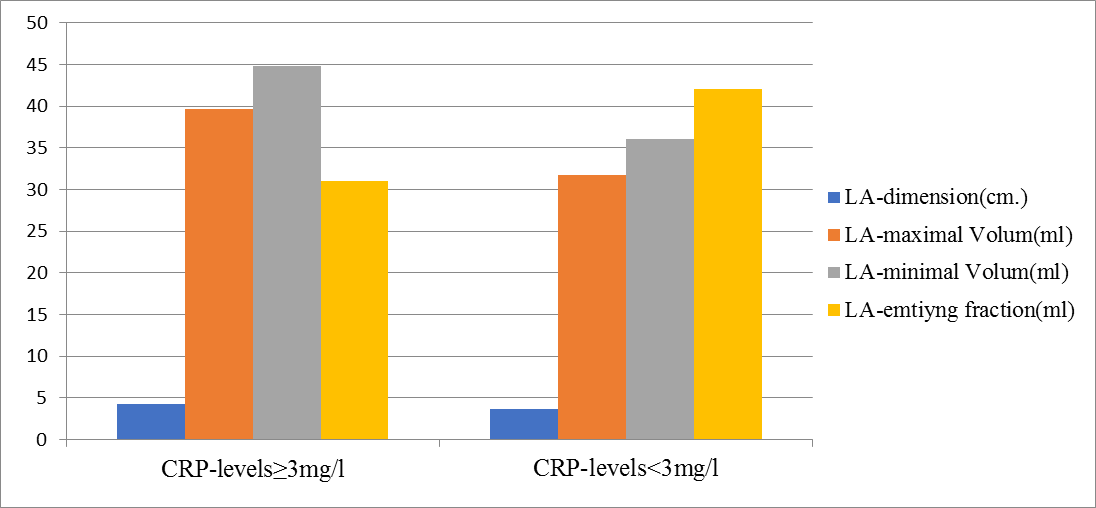
Figure 2: Left Atrial dimension and phasic function according CRP-levels.Dimension of LA,maximal volum and minimal volum,significantly increase among those with higher CRP-lvels.Whereas Left Atrial emtiyng fraction significantly decrease among those with higher CRP-levels.
Patients who had CRP levels above the cut-off of 3 mg/l,had increased dimension of LA (4.2±0.3 vs. 3.7±0.2,p=0.000),higher prevalence of enlargement of LA {defined as LAVI ≥29ml/m2, (84.1% vs.48.5%,p=0.002) and there was inverse relationship between LA function(defined as LAEF <45%) accordin to CRP levels.Patients who had CRP levels above the cut-off of 3 mg/l,had significantly reduced LAEF (30.8±3.4 vs.41.9±2.6, p=0.00).Parti- cipans with CRP levels above the cut-off of 3 mg/l, have significantly higher LAVI-maximal volum(39,6±2.9 vs. 31.7±1.6 , p=0.000) and higher LA.min.vol.(44.8±6.2 vs.36.1±3.6,p=0.000). Prevalence of LAVI>29 + reduced LAEF,was significantly higer in participans with CRP levels above the cut-off of 3 mg/l(74.4% vs. 43.9%,p=0.001),but there was inverse relation of normal LAVI + normal LAEF{prevalence of normal LAVI + normal LAEF,was significantly lower in participans with CRP levels above the cut-off of 3 mg/l(3.8% vs.17.2% p=0.001)}.(Figure.3).

Figure 3: Left Atrial volums and phasic function according CRP-levels.Frequence of Increased LA-volum index >29ml/m2,dereased LA emtyinfractio,was significantly higher in participans with higher CRP-levels.Frquence of normal LA volum index + normal LA emptying fraction were significantly lower in participans with higher CRP-levels.Whereas frequence of normal LA volum index+ reduced LA emptying fraction were significantly higher in participans with lower CRP-levels.
Significant difference between group was obsedrved in prevalence of LAVI normal + reduced LAEF. Prevalence of normal LAVI + reduced LAEF,was significantly lower in participans with CRP levels above the cut-off of 3 mg/l(14.2% vs. 34.1%,p=0.002).(Figure.3).
Association of CRP levels above the cut-off of 3 mg/l with type of AF(persistens and permanent) and features of cardiac structures and function, are presented in Table 3.
|
caracteristics |
OR |
Significance |
95% CI for Exp (B) |
|
|
Lower |
Upper |
|||
|
Persistent-AF |
8.824
|
.000
|
1.689
|
46.100
|
|
Permanent-AF
|
13.955 |
.000 |
2.676 |
72.780 |
|
LA.dimension
|
3.817 |
.000
|
0.989 |
1.544 |
|
LAVI >29ml/m2
|
4.014 |
.000 |
2.620 |
6.152 |
|
LAEF<45%
|
3.323 |
.000 |
2.062 |
5.351 |
|
LAVI>29 +LAEF-r
|
3.354 |
.000 |
1.693 |
6.646 |
Persistent-AF:persistent Atrial Fibrillation;Permanent Atrial Fibrillation; LAEF-r; Reduced left atrial emptying fraction.
Table 3: Logistic Regresion Model.Assotiation of CRP levels ≥ 3mg/l, with: Type of AF(persistens and permanent) and features of cardiac structures and function (LA.diemnsion, LAVI >29ml/m2, LAEF<45%, LAVI>29 +LAEF-r).
There was observed significant association of CRP levels above the cut-off of 3 mg/l with prevalence of persistent AF(OR=8.824,95% CI 1.689-46.100) and permanent AF(OR=13.955, 95%CI 2.676 -72.780).There was observed significant association between CRP levels above the cut-off of 3 mg/l and:increased LA dimension (OR=3.817,95% CI 0.989 -1.544),decreased LA function expressed by LAVI >29ml/m2(OR=4.014, 95% CI 2.620-6.152);LAEF <45%(OR=3.323,95%CI 2.062 -5.351) and LAVI>29 + LAEF-r (OR=3.354,95% CI 1.693 - 6.646).
Discusion:
In the present study we observed that, frequency of permanent type of AF among participans with MS and CRP levels above the cut-off of 3 mg/l ,was significantly higher than among participans with MS and CRP levels below 3 mg/l.Whereas frequency of paroxysmal type of AF was significantly higher in participans with MS and CRP levels below 3 mg/l,indicating different impact degree of systemic inflammation(assessed acrording to CRP levels), on the type of AF. Results that confirmed our hypothesis. Results that suggests the possibilities that, degree of systemic inflammation may be more proarrhythmic. Several study demonstrated that, the level of specific inflammatory biomarkers may provide information regarding of the AF duration [26,27,28]. Thus, if inflammation plays a causal role, it may be more pathogenetic in promoting persistence rather than initiation of AF. Nevertheless, the initiating and sustaining factors for AF may be different, and inflammation has been associated with both. Experimental and clinical data indicate that inflammation have been involved in the pathogenesis of both, metabolic syndrome and atrial fibrillation [6]. This makes inflammation one of many possible cofactors of AF. In perpetuating AF, inflammation might participate in the structural and electrical remodeling, inducing cellular degeneration, apoptosis, and subsequent atrial fibrosis and dilation. There was a corresponding trend towards a higher left ventricular mass index and permanent AF, in those with CRP levels above the cut-off of 3 mg/l in present study. Watanabe and co-authors found that left venricular mas, left ventricular end-systolic diameter and left atrial diameter were predictor of elevated CRP and persistent AF [29]. Simaliarly, Psychari and colleagues, in patients with persistensAF and permanent AF, showed that CRP were positively related to left atrial diameter and AF duration [30]. In our study, Left atrial size, as determined by LA dimension and LAVI, increased across AF types of paroxysmal, persistent, and permanent AF having enlarged LA. Similar Left atrial function, assessed by EF, significantly declined across the spectrum of AF types. Similar results has been found by other authors [31]. It is likely that inflammation is involved during electrical remodeling, in particular by increasing the inhomogeneity of atrial conduction. Moreover, inflammation seems to have variable effects on different types and duration of AF.
In our study we found that prevalence of normal left atrial dimension and impaered LA fungtion was higher in participans whith CRP levels below 3 mg/l than in participans with CRP levels above the cut-off of 3 mg/l.These finding suggests that: impaired LA function and electrical remodeling, might precede structural remodeling and the different levels of C-reactive protein have variable inpacts on the: degree of LA structural and functional remodeling in the patient with MS. Similar results has been found by others studies [32,33].It is well known that inflammatory processes have been associated with AF, but is uncertain whether this represents the couse or the effect of inflammation. Although mechanisms by which AF cause inflammation are unknown [34].
Future Directions: Understanding the pathogenesis of AF and the relationships between inflammation and AF is of both academic and clinical interest, because insights might lead to better prevention and treatment of this common but dangerous dysrhythmia. The detailed imaging of inflammation by positron emission tomographic or molecular means might help to clarify the role of inflammation in AF, and microRNA could prove to be of substantial value in the study of tissue and circulating biomarkers. If and when a causal link between inflammation and AF is proved, novel approaches to targeting inflammation, might lead to better therapeutic options.
While we evaluated a relatively large contemporary AF population, limitations should be noted. The cross-sectional design precludes the assessment of causal relationships between cardiac structure and function and AF type. The irregular rhythm of AF may lead to beat-to-beat variability in echocardiographic measurements; however, our assessments were averaged over multiple cardiac cycles. Assessing LA deformation and function with strain imaging may provide additional information and is a future direction. While we cannot exclude misclassification bias with respect to AF type, as paroxysmal, persistent, and permanent designations were determined by site investigators, our results are largely consistent with registries of AF patients [35].
Conclusions: We proved the hypothesis that different degree of inflammation have variable inpact on different types and duration of Atrial fibrillation, atrial functional and structural remodeling in the patient with MS.
These findings might lend further insight into impact of inflammation on clinical presentation of Atrial Fibrillation, atrial functional and structural remodeling in patients with Metabolic Syndrome. While a clear causal link between AF and inflammation has yet to be determined, anti-inflammatory therapeutic strategies may be a next logical step in AF care, one not burdened by proarrhythmic risk.
Conflict of interest: The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potencial confict of interest.