Clinical Cardiology Interventions
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2836-077X | Journal DOI: 10.61148/2836-077X/JCCI
Rajesh Vijayvergiya1*, Umesh Chandel1, Saroj K Sinha, Sonal Sangwan 2, Sakshi Mehta2, Veena Dhawan 2*
1Department of Cardiology, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India.
2Department of Experimental Medicine and Biotechnology, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
*Correspondence Author: Rajesh Vijayvergiya, Professor, Department of Cardiology, Faculty Offices, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh-160012, INDIA.
Received date: November 26, 2021
Accepted date: December 05, 2021
published date: December 15, 2021
Citation: Vijayvergiya R, Chandel U, Saroj K Sinha, Sangwan S, Mehta S, Dhawan V. (2021) “Effect of Proton Pump Inhibitors on Antiplatelet Function of Clopidogrel in Coronary Artery Disease Patients- An Indian Experience.” J Clinical Cardiology Interventions, 2(4); DOI: http;//doi.org/04.2021/1.1022
Copyright: © 2021 Rajesh Vijayvergiya. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Background: Cardiovascular diseases are the most common cause of deaths worldwide. Data shows that Indians are more predisposed to coronary artery disease (CAD) compared to other populations. Dual antiplatelet therapy with aspirin and Clopidogrel is recommended in the National and International guidelines for patients with ACS and/or PCI, Unfortunately, increased risk of upper gastrointestinal (GI) bleeding is associated. Therefore, patients on aspirin and clopidogrel are prescribed proton pump inhibitor (PPI) to counter GI bleeding. However, clopidogrel and PPI share the same metabolising enzyme CYP2C19, thereby, reducing the biologically active clopidogrel, and thus increasing the risk of myocardial infarction. Different PPIs have different affinity towards the enzyme and cytochrome polymorphisms play important roles in a patient’s pharmacological response to clopidogrel and PPI. Aim: The present study analyzes the effect of three PPIs namely Esomeprazole, Pantoprazole and Rabeprazole on the antiplatelet aggregation function of clopidogrel in North Indian subjects. Methods: Ninety patients (age 25-75 years) with angiography proven stable CAD on their daily dose of Aspirin (150 mg) and Clopidogrel (75mg) and other standard drugs were enrolled. These subjects were divided into 3 sub-groups with 30 subjects each and prescribed oral Esomeprazole (20mg), Pantoprazole (40mg) and Rabeprazole (20mg) twice a day respectively. Baseline platelet aggregation was recorded and then repeated after 4 weeks of combined antiplatelet and PPI therapy. Results: Our results demonstrated that none of the studied PPIs had any inhibitory or competitive effect on bioactivity of clopidogrel in the study subjects and that all the three PPIs can be used safely in North Indian CAD patients on Clopidogrel therapy. Conclusion: The present study suggests that all the three PPIs namely esomeprazole, pantoprazole and rabeprazole can be used safely in North Indian CAD patients on dual platelet therapy i.e. Clopidogrel and aspirin therapy.
Introduction
Aggregation of platelets and their activation leads to the generation of occlusive thrombus at the site of coronary arterial plaque rupture [1] . Thus, patients with stroke, myocardial infarction or peripheral artery disease routinely receive anti-platelet therapy as a part of standard medical management. Clopidogrel, an oral, thyropyridine-class antiplatelet agent, is extensively used to prevent the stent thrombosis after implantation of bare metal and drug-eluting stents in patients undergoing percutaneous coronary intervention (PCI). Dual anti-platelet therapy with aspirin and clopidogrel is recommended as standard medical care for patients with acute coronary syndrome or those undergoing PCI [2] . However, along with the reduction of adverse cardiovascular events, the dual antiplatelet therapy increases the risk of upper gastrointestinal bleeding [1,3-7] . Damaged gastric epithelium following inhibition of prostaglandin production by dual antiplatelet therapy leads to ulcerations and gastrointestinal bleeding [4,7,8] . Data from various studies have confirmed a significant reduction in the risk of GI bleeding and ulcers with the use of Proton Pump Inhibitors (PPI) in patients taking dual antiplatelet therapy. Therefore, many guidelines recommend adoption of gastroprotective strategies such as use of PPI along with antiplatelet therapy in order to reduce the risk of gastrointestinal bleeding [7, 9,10, 12-14] .
Clopidogrel is a pro-drug that is metabolized in liver by CYP2C19; isoenzyme of cytochrome P450 (CYP450), to generate its active metabolite. The plasma concentration of active metabolite of clopidogrel is dependent on the catalytic activity of CYP2C19 [15,16]. Metabolically bioactive clopidogrel specifically and irreversibly binds to platelet ADP receptor P2Y12 and consequently suppresses ADP induced platelet activation and aggregation [17] . PPIs are also metabolised mainly by CYP2C19 [18] . Therefore, concomitant administration of PPI and clopidogrel induces a drug-drug interaction that competitively inhibits the conversion of clopidogrel to its active metabolite, thereby reducing its plasma concentration and increasing the risk of re-infarction and/or stent thrombosis [18] . Studies have confirmed that different PPIs have variable affinity for CYP2C19. While omeprazole is a strong affinity substrate to CYP2C19, lansoprazole has weaker affinity as compared to omeprazole, whereas, rabeprazole has least association with CYP2C19 [19] . Contradictory and conflicting pharmacodynamic and clinical association studies data exists for lansoprazole, pantoprazole and esomeprazole [20, 21] .
Inter-individual differences in the antiplatelet activity of clopidogrel have been seen among different CYP2C19 genotype groups [15,16,22] . However, the association of concomitant administration of dual antiplatelet therapy and PPI in increasing the risk of major adverse cardiovascular events (MACE) in PCI patients remains controversial [13, 23-31] . The present study was carried out to evaluate the effect of commonly used PPIs namely esomeprazole, pantoprazole and rabeprazole on the antiplatelet function of clopidogrel in the North Indian population of coronary artery disease patients.
Methods
The study was carried out in the Department of Cardiology and Experimental Medicine & Biotechnology of the institute. A written informed consent was obtained from each of the subjects before their participation in the study.The trial was registered with www.clinicaltrials.gov (CTRI/2013/10/004067). The study protocol was approved by Institute’s Ethics Committee.
Ninety patients in the age group of 25 - 75 years and angiography proven CAD with stable disease were enrolled in the study. All these patients were on the daily dose of aspirin 150 mg and clopidogrel 75 mg, along with other drugs such as β-blockers, angiotensin converting enzyme inhibitors, statins etc. Patients of acute coronary syndrome, acute myocardial infarction or unstable angina, peptic ulcer disease, hereditary or acquired bleeding disorders, thrombocytopenia, chronic liver or kidney disease, pregnancy and on anticoagulation or PPI were excluded from the study. Baseline platelet aggregation for clopidogrel was analysed in the samples from the study groups by ADP-induced Light Transmission Aggregometer method. Out of total 90 subjects, a group of 30 subjects was made for each PPI namely esomeprazole, pantoprazole and rabeprazole respectively. They were grouped as per the computer generated random number. The oral doses of individual PPI used were as follows: esomeprazole 20 mg twice daily, Pantoprazole 40 mg twice daily and rabeprazole 20 mg twice daily. PPIs were given empty stomach in the morning and in evening. After 4 weeks of combined antiplatelet and PPI therapy, all patients had a repeat platelet aggregation study by the aforementioned method.
Platelet Aggregometry Method:
Platelet function was analyzed by ADP-induced Light Transmission Aggregometry method as described by Sibbing [32] . ADP induced platelet aggregometry is a widely used method to measure the responsiveness to clopidogrel. The system detects the electrical impedance change due to the adhesion and aggregation of platelets on two independent electrode-set surfaces in the test cuvette. A 2:1 solution of whole blood anticoagulated with sodium citrate and 0.9% NaCl was stirred at 37 ºC for 3 minutes in the test cuvette. 10 µmol/L ADP was added and the increase in electrical impedance was recorded continuously for 6 minutes. Mean value of two independent determinations was expressed as area under the curve (AUC) of the aggregation tracing [32] .
Statistical Analysis:
Quantitative data was presented as mean ±SD. Normality of quantitative data was checked by measures of Kolmogorov-Smirnov tests of normality. Comparisons of normally distributed continuous variables between 3 groups were done by One-Way ANOVA followed by Post- Hoc multiple comparisons. For skewed data, Kruskal Wallis test was applied. Mann-Whitney U-test was used for statistical analysis of skewed continuous variables for two groups. For time related variables before PPI value (AUC) and after PPI value (AUC), Wilcoxon signed rank test was applied (data for this variable was skewed). All calculations were two sided and performed using SPSS version 17 (Statistical packages for Social Sciences, Chicago, IL). p <0.05 was considered to be statistically significant.
Results
Baseline characteristics: The baseline characteristics of the study subjects are shown in Table 1 & 2. Out of a total of 121 patients enrolled earlier, 14 patients were lost to follow-up. Thus a total number of 90 study subjects were enrolled in the study. Out of these 80% were males and 20% were females. 23% were diabetics, 62.2% were hypertensives and 8.9% patients were found to be obese. Dyslipidemia was prevalent among 13.3% subjects and 30% had a family history of CAD. 63% of the subjects were alcoholics and 46.7% were smokers. Their prevalence in different groups of patients receiving oral PPIs is shown in Table 1 & 2.

Table 1: Baseline Clinical Parameters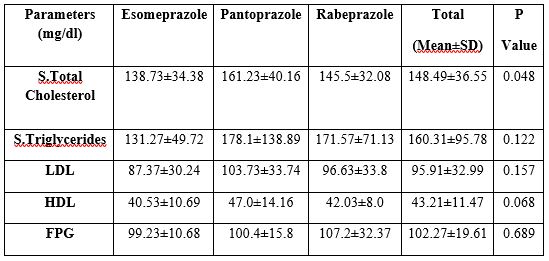
Table 2: Baseline lipid profile
Effect of concomitant dose of PPIs on lipids and lipoprotein profile:
The observations of the baseline lipid and lipoprotein profile demonstrated that for lipid profile in 3 groups, the difference was significant (p <.05) for total cholesterol whereas it was statistically insignificant for serum triglycerides, LDL and HDL levels (p>.05). Post hoc test was then applied which revealed statistically significant difference in cholesterol values in esomeprazole and Pantoprazole group (p>.05). However, Fasting Blood Sugar showed no significant difference among the groups (p>.05).
Effect of concomitant dose of PPIs on anti-platelet function of clopidogrel.
Mean platelet counts was 2.06 x 105 for esomeprazole, 2.28 x 105 for pantoprazole and 2.09 x 105 for rabeprazole respectively, which was not different among the three groups (Fig. 1).
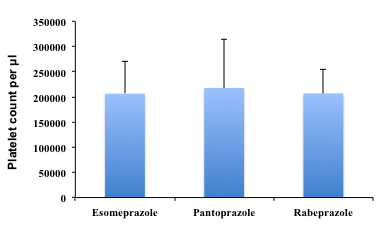
Platelet count was determined from the blood sample taken from patients according to the standard protocol. Each vertical bar represents the mean platelet count for respective group ± SD.
Fig. 1: Mean Platelet count per µl.
Mean value of platelet aggregation (MPA) at baseline was 45.75 ± 22.25 in esomeprazole, 42.50 ± 22.14 in pantoprazole and 45.30 ± 29.46 in rabeprazole group. (Fig. 2). Thus, after applying the Kruskal wallis test, the baseline mean platelet aggregation values too were not significantly different in the 3 groups (p = 0.05) (Fig. 2).

MPA (Mean Platelet Aggregation) values were calculated at baseline and 4 weeks after PPI therapy according to ADP induced Light Transmission Aggregometry method as described by Sibbing. Each vertical bar represents the MPA for respective group. Blue bars indicate MPA value at baseline; Red bars indicate MPA value Post PPI.
Figure 2: Mean Platelet Aggregation Value
Patients were given PPIs randomly and followed up after 4 weeks. MPA value after 4 weeks in esomeprazole group rose up to 47.63 ± 22.14. However, in pantoprazole and rabeprazole group, a decrease in MPA was observed, the values being 35.03 ±18.40 and 38.30 ± 26.06 respectively (Fig. 2). Difference between baseline and post PPI values in esomeprazole came out to be statistically insignificant as calculated by Wilcoxon test (p = 0.09). Pantoprazole and rabeprazole groups showed a significant difference as compared to their respective baseline values which is difficult to be explained (p < 0.001). Probably the difference in time interval in processing baseline and post PPI blood samples could be the reason for observed results in these groups.
While applying the ANOVA and post hoc tests in platelet aggregation values between the three groups, data revealed that esomeprazole group was significantly different from the two other groups (p = 0.001). No significant difference was observed among pantoprazole group and rabeprazole receiving patients (p > 0.05).
Discussion
The PPIs are often prescribed for the prophylaxis of serious upper GI bleeding complications when DAT is used to prevent recurrent MACE in CAD patients. Clinical studies have demonstrated an increased risk of MACE in patients using clopidogrel and PPI, simultaneously [13, 23-31] . Clopidogrel and PPI interact at the level of clopidogrel metabolising enzyme CYP2C19 and thus outcome of clopidogrel-PPI interaction varies with varying CYP2C19 genotypes. Individuals having allele CYP2C19 *2 and/or CYP2C19 *3 are weak metabolizers of clopidogrel and thus shift from ‘responders’ to ‘non-responders’ when placed on a treatment with clopidogrel + PPI [18] .
A meta-analysis has shown increased risk of adverse outcomes when patients are prescribed a PPI in conjunction with clopidogrel [33] . A very interesting study revealed that efficacy of clopidogrel was unaffected by PPI use in CREDO (Clopidogrel for reduction of events during observation) while was found to be harmful when used simultaneously with PPI in CAPRIE (clopidogrel versus aspirin in patients at risk of ischemic events) [34] . A study of 124 patients undergoing elective stent implantation showed attenuation in inhibitory effect of clopidogrel when used with omeprazole [35] . Another study of around 3000 patients ruled out any cardiovascular interaction between clopidogrel and omeprazole [12] , however a recent report has recommended pantoprazole; known to have a weaker association with CYP2C19, as a better treatment option over strong inhibitor omeprazole [36] . A 5-year retrospective cohort analysis of around six thousand Chinese patients suggested an increased risk for developing MACE while using DAT and PPI concomitantly [37] however this study does not specify various PPIs used and their effects individually.
In this study, Mean Platelet Aggregation value was increased by esomeprazole but was not statistically significant, thereby suggesting no significant inhibitory effect of esomeprazole on clopidogrel. Interestingly, Mean Platelet aggregation values decreased after pantoprazole and rabeprazole administration. However, it is very difficult to explain this significant decrease in MPA values in these groups; nonetheless, this implies that they too do not have inhibitory effect on antiplatelet function of clopidogrel. Our data is supported by similar studies reported in the literature. A study done on 300 CAD patients by Siller-Matula et al. also concluded that esomeprazole and pantoprazole are not associated with the impaired response to clopidogrel [38]. Another study done on about 2500 French MI patients concludes that use of pantoprazole, esomeprazole, lansoprazole and omeprazole does not increase the risk of cardiovascular events and mortality [39] also supports our conclusion of pantoprazole, esomeprazole and rabeprazole not affecting the bio-activity of clopidogrel adversely.
Since PPI-induced risk reduction clearly outweighs the cardiovascular risks in patients because of their use, the present study suggests that all the three PPIs namely esomeprazole, pantoprazole and rabeprazole can be used safely in North Indian CAD patients on clopidogrel therapy. However, if at all, pantoprazole and rabeprazole should be preferred over esomeprazole.
Conflicts of Interest
All authors have read the journal’s policy on disclosure of potential conflicts of interest and none to declare.
Funding
The study was supported by grant received from Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India.
Institutional review board statement:
The study was reviewed and approved by the Institutional Review Board of Post Graduate Institute of Medical Education and Research (PGIMER) ,Chandigarh, India.
Core Tip: