Clinical Cardiology Interventions
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2836-077X | Journal DOI: 10.61148/2836-077X/JCCI
Ahsan A Khan, Sunil James, Mengshi Yuan, Latoya Woolery, Nina Huppertz, Mushidur Rahman, Chetan Varma, Stavros Apostolakis, Vinoda Sharma *
Department of Cardiology, Sandwell & West Birmingham Hospitals NHS Trust, Birmingham, United Kingdom
*Corresponding Author: Vinoda Sharma, Department of Cardiology, Sandwell & West Birmingham Hospitals NHS Trust, Birmingham, United Kingdom
Received date: June 22, 2021
Accepted date: June 29, 2021
Published date: August 04, 2021
Citation: Ahsan A Khan, James S, Yuan M, Woolery L, Huppertz N. (2021) “Observations on Echocardiographic Findings in Patients with Covid-19.” J Clinical Cardiology Interventions, 2(3); DOI: http;//doi.org/04.2021/1.1019
Copyright: © 2021 Vinoda Sharma. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Background: The novel coronavirus (SARS-CoV-2) has created global havoc by causing Coronavirus Disease 2019 (COVID-19). Cardiovascular involvement and presentation varies but there is limited data on echocardiographic findings. We aimed to analyse on echocardiographic details in patients with COVID-19.
Methods: Retrospective analysis was undertaken of all patients who were admitted to Sandwell and West Birmingham (SWBH) Hospitals NHS Trust with COVID-19 from March 2020 to May 2020 and underwent a transthoracic echocardiogram (TTE). Data regarding demographics, clinical presentation, TTE findings, clinical course and outcomes were collected. Patients were divided into two groups (abnormal TTE and normal TTE).
Results: 66 out of 461 patients with COVID-19 had a TTE in our trust. 46 patients (69.7%) had abnormal findings on their TTE. Tricuspid regurgitation was the most common abnormality seen on TTE (26 (56.5%) patients), followed by aortic regurgitation (13 (28.3%) patients) and mitral regurgitation (12 (26.1%) patients). Patients with an abnormal TTE more frequently had increased probability of PHTN (none: 47.8% versus 100%, p value for trend=0.0002) and were also more likely to have raised LDH>255 (76.3% vs 23.7%, p=0.006) with low haemoglobin (115 ± 24 g/L vs 129 ± 26 g/L, p= 0.03). Significantly more patients in the abnormal TTE group died during their inpatient stay (p = 0.01).
Conclusions: This is a detailed observational study looking at echocardiographic changes in COVID-19 patients. The most common abnormality was valve regurgitation. Patients with abnormal TTE were more likely to die in hospital and such patients require close surveillance. Page 3 of 24
Introduction
Viral pneumonias have recently emerged as a great threat to global public health.1 Most recently, the novel coronavirus (SARS-CoV-2), known to cause severe pneumonia has reaped global havoc affecting even the most substantial health care systems in the world. As with most viral pneumonias, a strong relationship between cardiovascular disease and COVID-19 has been noted.1 More specifically, COVID-19 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a single-stranded RNA virus, has its deleterious effect by binding to the human angiotensin-converting enzyme 2 (ACE2) receptor expressed in alveolar cells, vascular endothelium, intestinal epithelial cells, kidneys, and the heart.2-4 Furthermore,
Between 12-22% of COVID-19 patients admitted to hospital have diabetes and up to 30% have hypertension.5-7 High levels of ACE2 have been noted in patients with diabetes and/or hypertension treated with ACE inhibitors 6, 7
It has been, previously, well characterised that myocardial injury, demonstrated by elevated high-sensitivity cardiac troponin I makers is seen in patients with COVID-19 infection.8 In fact, cardiac injury was observed to confer a high risk of mortality for such inpatients.9 Although, the mechanistic awareness of how SARS-CoV-2 causes myocardial damage is still unknown; hypoxaemia induced pulmonary hypertension (PHTN), systemic inflammation resulting in increased afterload and thus, worsening cardiac function and direct invasion of the virus into the myocardium resulting in myocarditis are possible mechanisms of injury.1, 10
There is at present insufficient data to determine the cause or consequence of cardiac injury. Some have stipulated various consequences and molecular mechanisms, nothing of Page 5 of 24
which is substantial or causal.8, 10 Although many studies have examined the potential of troponin I and other biomarkers as a marker of severity, there are very few studies, to our knowledge, that have examined the echocardiography findings in COVID-19 patients.11-13 Complicating this, is the difficulty of performing echocardiography at bedside whilst using personal protective equipment and the risk of infection to staff from enhanced exposure to this virus. Thus, observation of cardiac involvement in a functional manner with echocardiography has been limited. As such, we report our retrospective echocardiography findings of COVID-19 patients.
Methods
We carried out a retrospective, observational study of COVID-19 positive patients admitted to Sandwell and West Birmingham (SWBH) Hospitals NHS Trust from March 2020 to May 2020 who underwent a transthoracic echocardiogram (TTE). Patients included were either admitted with symptoms of COVID-19 infection or had a positive swab result whilst already an in-patient with an alternative diagnosis. The study was approved by the local audit department (reference number: 1176) and followed the principles of the Declaration of Helsinki. The study also adhered to “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies” as well as the “Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD)” guidelines.14-16
Data was extracted from the directorate’s daily COVID-19 report database by members of the Cardiology Department. The following patients were excluded from the analysis: age <18 years old, outpatients who were COVID-19 positive (in the community), patients Page 6 of 24 admitted to other hospitals, and patients who did not have an echocardiogram. All TTE scans were performed by experienced, British Society of Echocardiography (BSE) accredited sonographers.
Echocardiographic Methodology:
Our department followed British Society of Echocardiography (BSE) guidance for performing echocardiograms during COVID-19. All echocardiographers wore full Personal Protective Equipment (PPE) for all COVID-19 positive and suspected patients, which included a fit-tested FFP3 mask, face visor or goggles, long-sleeved fluid resistant gown, disposable gloves, and scrubs. A dedicated echo machine was used, and all echocardiograms were performed at the bedside. ECG leads and electrodes were stored in a draw on echo machine to prevent contamination. ECG leads were not applied to COVID-19 positive or suspected patients in accordance with BSE clinical guidance regarding provision of transthoracic echocardiography during the COVID-19 pandemic17 meaning that time loops were stored as opposed to ECG loops/cycles. Echo images were stored, and parameters were measured offline to minimise time spent in high viral load areas. This guidance also suggested that all echocardiograms on COVID-19 positive or suspected patients should be limited to answering the clinical question with a Level 1 BSE scan 18 sufficient in most cases giving an assessment of biventricular systolic function and major structural valve disease. If the echocardiogram performed using the Level 1 BSE minimum dataset displayed evidence of any significant abnormalities, then departmental protocol states that a complete BSE standard assessment should be attempted where image quality allows, during the COVID-19 pandemic the images could be stored and measured offline to minimise time spent in high viral load areas. Page 7 of 24
Echocardiography definitions of abnormalities (supplementary table 1):
|
LV FUNCTION |
|
|
RV FUNCTION |
|
|
RV SIZE |
|
|
ATRIAL SIZE |
|
|
PERICARDIAL EFFUSION |
|
|
AORTIC STENOSIS |
|
|
AORTIC REGURGITATION |
|
|
MITRAL REGURGITATION |
|
|
TRICUSPID REGURGITATION |
|
|
PULMONARY REGURGITATION |
|
Supplementary Table1: Variables used to define the echocardiographic pathologies
To determine LV function, an LV ejection fraction (LVEF) by the Simpson’s Biplane method was attempted if image quality allowed. When unable to do so, a visual LVEF range was provided by the sonographer. RV function was determined by performing either a tricuspid annular plane systolic excursion (TAPSE), RV peak systolic S wave velocity (RV S’) using TDI, and/or a fractional area change (FAC) percentage. When unable to perform measurements due to image quality, a visual assessment was made by the sonographer.
Abnormal echocardiogram was defined as any one of the following:
Patients were divided into 2 groups, those with abnormal echocardiogram and those with normal echocardiogram. In addition to the echocardiographic data, information on baseline clinical characteristics including age, sex, ethnicity, height, weight, body mass index (BMI), co-morbidities and smoking status was also collected. Biomarkers associated with poor prognosis in COVID-19 patients were also checked and included in the analysis. These include full blood count, kidney function using estimated Glomerular Filtrate Rate (eGFR), troponin I, D-Dimer, Lactate Dehydrogenase (LDH), ferritin, magnesium and procalcitonin. Patients’ 12 lead ECG on admission was also reviewed. Information on patients’ outcome including death was also collected. Severity of COVID-19 infection was classified as Page 8 of 24
asymptomatic, mild, moderate, severe, and critical based on published definitions19, 20. Information on whether the patients required ventilation was also collected. Patients in the two groups were compared for baseline characteristics, biomarkers, severity of COVID-19 infection, ECG changes and in-hospital mortality.
Statistical Analysis
Descriptive statistics are presented as mean standard deviation (SD) or median with interquartile range, as appropriate for continuous variables. Categorical variables are expressed as numbers and percentages. Statistical analysis was performed using SPSS software, version 26 (SPSS Inc., Chicago, Illinois). Continuous variables were tested for normality using the Shapiro-Wilk test. Non-normally distributed data were logarithmically transformed, and the distribution re-checked with a Shapiro-Wilk test. If passed, data was analysed using an independent Student’s t-test. Data that were still not normally distributed were analysed with the Mann-Whitney U test using original non-log transformed data. A p value of <0.05 was considered statistically significant.
Results
461 patients in our trust between March and May 2020 with COVID-19 were screened of whom 66 had a TTE. 46 patients (69.7%) had abnormal findings on their TTE. Patients in the two groups, abnormal TTE and normal TTE were well matched for age, sex, ethnicity, and clinical characteristics (table 1).
|
Variable |
Abnormal TTE (n = 46) |
Normal TTE (n = 20) |
Significance p value |
|
Age (mean ± SD) |
63 ± 14 |
60 ± 18 |
0.38 |
|
Sex Male, n (%) Female, n (%) |
26 (56.5%) 20 (43.5%) |
12 (60%) 8 (40%) |
0.79 |
|
Ethnicity Caucasians, n (%) Asians, n (%) Blacks, n (%) Mixed, n (%) Unknown, n (%) |
18 (39.1%) 12 (26.1%) 2 (4.3%) 1 (2.2%) 13 (28.3%) |
9 (45%) 6 (30%) 2 (10%) 0 (0%) 3 (15%) |
-
|
|
Clinical characteristics |
|||
|
Height (m) |
1.67 ± 0.10 |
1.68 ± 0.09 |
0.75 |
|
Weight (kg) |
79.2 ± 1.2 |
84.3 ± 1.3 |
0.33 |
|
BMI (kg/m2) |
29.05 ± 5.97 |
31.49 ± 7.60 |
0.21 |
|
Hypertension, n (%) |
21 (45.7%) |
12 (60%) |
0.28 |
|
Diabetes Mellitus, n (%) |
18 (39.1%) |
7 (35%) |
0.75 |
|
COPD, n (%) |
6 (13%) |
2 (10%) |
1.00 |
|
Smoker, n (%) |
10 (21.7%) |
2 (10%) |
0.71 |
|
AF, n (%) |
6 (13.0%) |
0 (0%) |
0.17 |
|
Hypercholesterolemia, n (%) |
8 (17.4%) |
4 (20%) |
1.00 |
|
Previous IHD, n (%) |
5 (10.9%) |
2 (10%) |
1.00 |
|
Previous MI, n (%) |
1 (2.2%) |
1 (5%) |
0.52 |
|
Previous PCI, n (%) |
1 (2.2%) |
1 (5%) |
0.52 |
|
Previous CABG, n (%) |
0 (0%) |
0 (0%) |
- |
|
PVD, n (%) |
3 (6.5%) |
1 (5%) |
1.00 |
|
Severity of COVID-19 mild/asymptomatic moderate severe/critical |
1 (2.2%) 13 (28.3%) 31 (69.6%) |
4 (20%) 4 (20%) 12 (60%) |
p=0.04 |
|
Ventilated None Invasive Non-Invasive |
20 (43.5%) 20 (43.5%) 6 (13.0%) |
14 (70.0%) 5 (25.0%) 1 (5.0%) |
For trend p=0.06 |
|
Haemoglobin (g/L) |
115 ± 24 |
129 ± 26 |
0.03 |
|
White cell count (10*9/L) |
10.5 ± 1.6 |
9.4 ± 1.6 |
0.42 |
|
Lymphocytes (10*9/L) |
1.1 ± 1.9 |
1.2 ± 1.7 |
0.45 |
|
eGFR (ml/min/1.73m2) |
60 [40 – 83] |
74 [49 – 90] |
0.22 |
|
Hs Troponin I (ng/L) |
35 [10 – 203] |
16 [8 – 66] |
0.37 |
|
Highest Hs Troponin I (ng/L) |
313 ± 7 |
640 ± 19 |
0.66 |
|
D-Dimer (ugFEU/ml) |
8.3 ± 6 |
2.5 ± 3 |
0.21 |
|
LDH (U/L) |
544 ± 1 |
359 ± 2 |
0.004 |
|
LDH>255 |
76.3% |
23.7% |
0.006 |
|
Ferritin (mg/L) |
751 ± 3 |
618 ± 3 |
0.60 |
|
Magnesium (mmol/L) |
0.86 ± 1.21 |
0.86 ± 1.30 |
1.00 |
|
Procalcitonin |
0.89 ± 3.96 |
0.58 ± 4.59 |
0.54 |
|
ECG Rhythm Normal sinus rhythm AF/Atrial flutter Sinus tachycardia Bundle Branch Block AV block ST elevation ST depression T-wave inversion |
20 (43.5%) 4 (8.7%) 11 (23.9%) 4 (8.7%) 2 (4.3%) 2 (4.3%) 2 (4.3%) 9 (19.6%) |
10 (50%) 0 (0%) 9 (45%) 0 (0%) 0 (0%) 0 (0%) 1 (5%) 5 (25%) |
-
|
|
ECG Rate (bpm) |
95 ± 19 |
98 ± 17 |
0.61 |
|
Outcome |
|||
|
Death |
20 (43.5%) |
2 (10%) |
0.01 |
standard deviation (SD). Non-normally distributed data are displayed as median with interquartile ranges. Normality test was performed using Shapiro-Wilk test. Statistical differences were tested using an independent t-test for normally distributed data and Mann-Whitney U test for non-normally distributed data. Categorical data was compared using Chi-square test. Where Chi-square test was not valid, Fisher’s Exact Test was used. Significance p 0.05. – = unable to calculate p value as sample size too small/statistical test not valid. AF = atrial fibrillation; AV = atrioventricular; BMI = Body Mass Index; bpm = beats per minute; CABG = Coronary Artery Bypass Graft; COPD = Chronic Obstructive Pulmonary Disease; eGFR = estimated Glomerular Filtration Rate; IHD = Ischaemic Heart Disease; LDH = Lactate Dehydrogenase; MI = Myocardial Infarction; PCI = Primary Coronary Intervention; PVD = Peripheral vascular disease; TTE = Transthoracic echocardiogram
Table 1: Demographics and clinical characteristics
Shortness of breath, cough and fever were the most common presenting symptoms in our patients. Patients with abnormal TTE more often had more severe COVID-19 infection than those with normal TTE (asymptomatic/mild: 2.2%,
moderate: 28.3%, severe/critical: 69.6% vs asymptomatic/mild: 20%, moderate: 20%, severe/critical: 60%, p=0.04).
Patients in the abnormal TTE group had lower mean haemoglobin compared to the normal TTE group which was statistically significant (115 ± 24 vs 129 ± 26, p = 0.03). There were no significant differences in white cell count, lymphocytes, renal function, admission and highest troponins between the two groups. Mean LDH was noted to be significantly higher in the abnormal TTE group compared to normal TTE group (544 ± 1 vs 359 ± 2, p = 0.004). Patients with abnormal echocardiogram were more likely to have LDH cut off>255 (76.3% vs 23.7%, p=0.006). Also, patients with moderate or more severe COVID-19 disease had significantly raised LDH>255 (mean 536.7 ± 261.1, p< 0.0001). D-Dimer, ferritin, magnesium and procalcitonin levels were similar between the two groups l.
Majority of patients in both groups were in normal sinus rhythm on their 12 lead electrocardiograms (ECGs) on admission. However, 4 (8.7%) patients in the abnormal TTE group were found to be in atrial fibrillation/flutter whereas 0 patients in the normal TTE group. Similarly, there were 4 patients with bundle branch block, 2 with first degree AV block, 2 with ST elevation in abnormal TTE group compared to 0 in the normal TTE group. There were no significant differences in the mean heart rate on the 12 lead ECG between the two groups.
The echocardiogram findings of patients in the abnormal TTE group are detailed in table 2.
|
Variable |
Parameters |
Number of patients |
|
LV systolic function |
Normal |
34 |
|
Mildly impaired |
2 |
|
|
Moderately impaired |
2 |
|
|
Severely impaired |
4 |
|
|
Hyperdynamic |
4 |
|
|
LV Regional Wall Motion Abnormalities (RWMAs) |
Present |
4
|
|
Absent |
42 |
|
|
RV systolic function |
Normal |
34 |
|
Impaired |
12 |
|
|
Atrial size
|
Normal |
32 |
|
Dilated LA |
4 |
|
|
Dilated RA |
4 |
|
|
Bilateral dilatation |
6 |
|
|
Valvular disease
|
Present |
37 |
|
Absent |
9 |
|
|
Pericardial effusion |
Present |
4 |
|
Absent |
42 |
|
|
Pulmonary Artery Systolic Pressure (PASP) |
No TR so unable to measure |
42 |
|
Mean PASP |
47 ± 12 |
|
|
Probability of pulmonary hypertension |
None |
42 |
|
Low |
5 |
|
|
Intermediate |
8 |
|
|
High |
11 |
Table 2: Echocardiogram findings in abnormal TTE group
|
Reason for echo request
|
Number of patients |
|
Left Ventricular or Right Ventricular function assessment
|
46 |
|
Endocarditis
|
6 |
|
Cardiac arrest
|
1 |
|
Cardiomyopathy/myocarditis/heart failure
|
6 |
|
ECG changes
|
1 |
|
COVID positive
|
2 |
|
Stroke
|
2 |
|
Valvular assessment
|
5 |
Supplementary Table2: Reasons for echocardiogram request
Majority of these patients had evidence of valvular heart disease but no other pathology. Tricuspid regurgitation was the most common abnormality, seen in 26 (56.5%) patients, followed by aortic regurgitation (AR) (13 (28.3%) patients) and mitral regurgitation (MR) (12 Page 10 of 24
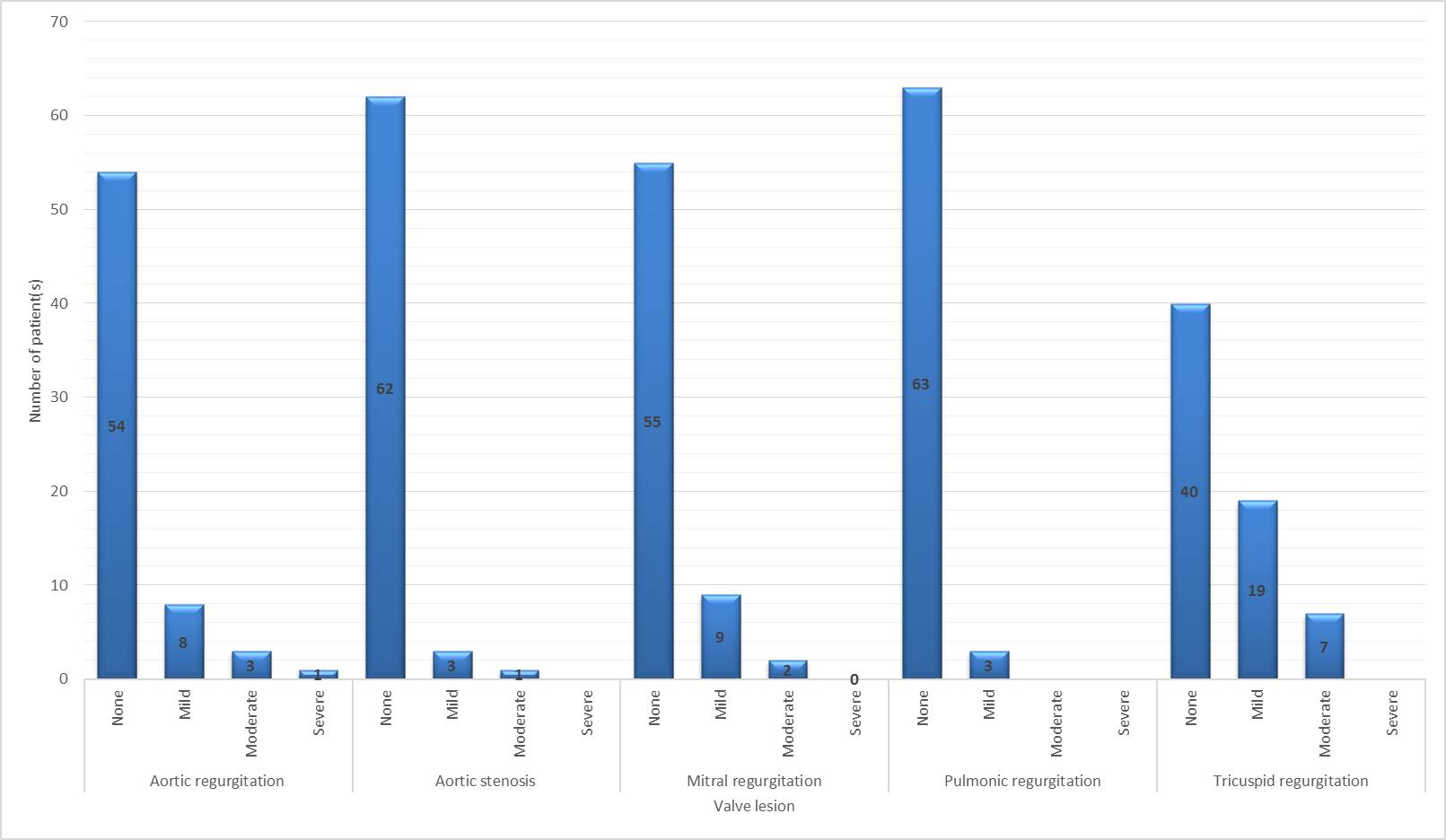
(26.1%) patients). Figure 1 describes the severity of each valvular lesion. The mean pulmonary artery systolic pressure (PASP) was found to be 47 ± 12 with majority of patients having mild PHTN. Probability of PHTN: 5 (7.5%) patients had low probability, 8 (12%) patients had intermediate probability, and 11 (16%) patients had high probability of pulmonary hypertension. Patients with an abnormal TTE more frequently had increased probability of PHTN (none: 47.8% versus 100%, p value for trend=0.0002, Supplementary table 3).
|
Variable |
Kendall’s tau |
95% CI |
Significance p value
|
|
Ventilation (none, invasive, non-invasive) versus probability of pulmonary hypertension (none, low, intermediate, high) |
0.214 |
-0.009 to 0.421 |
p=0.01 |
|
Variable
|
Abnormal TTE |
Normal TTE |
Significance p value |
|
Probability of pulmonary hypertension none low intermediate high |
22 (47.8%) 5 (10.9%) 8 (17.4%) 11 (23.9%) |
20 (100%) 0 0 0 |
p value for trend=0.0002 |
|
Variable |
Died |
Survivor |
Significance p value
|
|
Probability of pulmonary hypertension none low intermediate high |
10 (43.5%) 2 (8.7%) 5 (21.7%) 6 (26.1%) |
32 (74.4%) 3 (7.0%) 3 (7.0%) 5 (11.6%) |
p value for trend=0.01 |
|
Variable |
Number of patients |
Haemoglobin g/L mean (±SD) |
Significance p value
|
|
Probability of pulmonary hypertension none low intermediate high |
42 (63.6%) 5 (7.6%) 8 (12.1%) 11 (16.7%)
|
127.1 (±23.0) 97.2 (±18.5) 104.0 (±23.1) 111.2 (±26.8) |
p=0.005 |
Supplementary Table 3: Probability of pulmonary hypertension and ventilation, abnormal TTE and mortality
Also, patients with higher probability of PHTN were less likely to survive (died: none: 43.5% vs 74.4%; low: 8.7% vs 7%; intermediate:21.7% vs 7%, high: 26.1% vs 11.6%, p value for trend: 0.01, supplementary table 3).
Previous echocardiograms: Only 14% of the patients had a previous echocardiogram. With regards to valvular disease, there were 4 cases where the previous echocardiogram confirmed pre-existing valvular stenosis or regurgitation and one patient had pre-existing severe AR. There were 2 cases of valvular disease that progressed in severity and 5 cases of newly diagnosed valvular disease. In regard to PHTN, there was 1 case where the probability of PHTN progressed from low to high. There was also one patient newly diagnosed with intermediate probability of PHTN. There were 3 new cases of new RV dilatation that was not documented on a previous echo. 2 of these cases also reported new RV dysfunction. There was 1 patient with global LV hypokinesia resulting in a severely impaired LV function that previously had normal LV function.
Patients with an abnormal echocardiogram were more likely to be invasively ventilated (43.5% vs 25.0%, p=0.06, table 1). There was a weak correlation between ventilation and the probability of PHTN (Kendall’s tau 0.214, 95% CI -0.009 to 0.421, p=0.01, supplementary Page 11 of 24
table 3). Significantly more patients in the abnormal TTE group died during their hospital admission compared to normal TTE (p = 0.01).
Discussion
We present a detailed analysis of echocardiographic changes in patients with COVID-19 and relationship to biomarkers. Consistent with many previous studies, our data indicates that patients with abnormal TTE were more likely to die in hospital.11, 12, 21-23 Peng et al. have previously shown that abnormal echocardiography features were linked to the severity of disease and consequent cardiovascular sequalae.24 The aetiology of cardiac dysfunction as it relates to abnormal TTE is likely to be multifactorial. Although direct invasion of the virus into the myocardium is one reason; it is likely that in the majority of the cases, myocardial infarction (Type II mainly) due to reduced oxygen perfusion and respiratory failure, microangiopathy secondary to cytokine storm and stress cardiomyopathy are potential causes.5, 25-31
Patients with abnormal TTE were also more frequently on invasive ventilation. This probably reflects the severity of COVID-19 infection in these patients more than anything else 20.
The commonest echocardiogram abnormality noted in our study was valvular regurgitation of which TR was the most frequent, similar to findings from another study.32 This could be due to PHTN (secondary to pulmonary pathology) and or uni- or biventricular dysfunction.33 Approximately 26% of our patients had impaired right ventricle (RV) systolic dysfunction and 9% had right atrial dilatation. It is estimated that up to two-thirds of critically ill COVID-19 patients have acute respiratory distress syndrome which results in RV systolic dysfunction.5, 34, 35 In fact, patients with disproportionately enlarged RV and reduced left ventricle (LV) Page 12 of 24
systolic function needs to be monitored closely with early goal-directed therapies and has been shown to guide management of the critically ill.24, 36-38 Although RV dysfunction relates to poor outcomes, RV longitudinal strain is more highly predictive of mortality in COVID-19 patients.34, 39, 40 Despite not being overly sensitive for a pulmonary embolism (PE), echocardiographic features of right heart strain and right heart dysfunction can indicate the presence of a PE. This is an important finding as studies have observed venous thromboembolism in approximately quarter of their critically ill patients.41, 42 Despite the advantages of RV longitudinal strain, time constraints and increased risk of exposure to the SARS-CoV-2 virus for the sonographer limits its usage in the COVID-19 positive patients.
Our study corroborates with other literature which suggests that elevated PASP was related to morbidity.34 A case series also noted elevated PASP in their patients which could be explained by PHTN or recurrent pulmonary-embolic disease.36, 43 High PASP as it relates to PHTN, whether underlying or as a consequent result of SARS-CoV-2-related lung injury, pulmonary hypercoagulable state or cardiomyopathy confers significant mortality and morbidity.44, 45 Raised pulmonary pressure >35mmHg is an independent predictor of mortality in COVID-19 as demonstrated by Pagnesi et al 46. Another case report with COVID-19 fulminant myocarditis noticed an elevation of PASP and then a sudden depreciation in PASP likely linked to right heart functional decline secondary to sustained overload.47 In concordance with other studies, elevated PASP and right heart enlargement, was significantly predictive of mortality.32 In fact, taking into consideration the crucial role of right heart function, the German Society of Cardiology, concluded that optimum RV functioning was essential for COVID-19 patients’ prognosis.48 Page 13 of 24
Some of our patients already had a previous echocardiogram performed, however in the remainder, echocardiographic abnormalities could have been present before the infection and they may reflect the pre-existing condition making the infection more complicated, and perhaps leading to death.
In addition to TTE abnormalities, we found that LDH was significantly raised in our study in both those with an abnormal TTE and those with moderate or more severe COVID-19 disease. It is well known that raised LDH, although non-specific correlates well with cardiac dysfunction.49, 50 A recent study has demonstrated that elevated LDH levels >255 are associated with a 6 fold increase in developing severe disease and a 16 fold increase in mortality in COVID-19 patients 51. Furthermore, we noted that mean haemoglobin in patients with abnormal TTE was significantly lower than in patients with normal TTE. There appears to be no data that shows a relationship between SARS-CoV-2 and anaemia. However, one can hypothesise that as anaemia results in reduced oxygen perfusion, the consequent compensatory cardiovascular response can induce diastolic dysfunction and left ventricular remodelling observed on TTE.52, 53 Raised LDH may parallel fall in haemoglobin as indicating critical disease in COVID-19 patients. 54
Alongside abnormal TTE and lymphopenia, there is a growing body of evidence that relates obesity with poor outcomes amongst COVID-19 patients.55-57 BMI was raised in both our abnormal and normal TTE groups. Obesity results in increased cardiac output and has a detrimental effect on diastolic function; as such, any decompensation, in this case, caused by SARS-CoV-2 can augment existing cardiac dysfunction resulting to poor outcomes.58
Strengths and limitations Page 14 of 24
As far as we are aware, this is one of the studies to look at echocardiographic findings in detail in patients with COVID-19 and its relationship to several biomarkers. Participants in our groups were well-matched for age, sex, and comorbidities. Our study does have several limitations, such as modest sample sizes, raising the potential for a type II error, which may contribute to the small number of independent associations seen in our analysis. This is a retrospective observational study over a limited period from two linked hospital in a single centre. We have included patients who had already been hospitalised prior to their diagnosis of COVID-19 and therefore this may have been a confounding factor as their underlying primary diagnosis could have influenced their echocardiographic changes. Additionally, we cannot conclude that abnormal TTE findings are directly attributed to COVID-19. For definite conclusions, a larger cohort or multicentre analysis is required.
Conclusion
Patients with COVID-19 do have abnormal findings on TTE with valvular regurgitation being the most common. COVID-19 patients with abnormal TTE more frequently have elevated LDH and low haemoglobin, all three of which may be markers of disease severity. Patients with abnormal TTE and elevated PASP were more likely to die in hospital and such patients require close surveillance. Page 15 of 24
Declarations:
Ethical Approval and Consent to participate:
The study was approved by the local audit department (reference number: 1176) and followed the principles of the Declaration of Helsinki. The study also adhered to “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies” 15
Consent for publication:
As this was a retrospective study and with anonymised data, no formal consent was taken however this study was approved by the local audit department.
Availability of data and materials: Anonymised, select data is available on special request
Competing interests: None of the authors has any competing interests
Funding: Not applicable
Authors' contributions:
All authors have contributed to the manuscript in at least three of the following sections: Methodology, Formal analysis, Visualisation, Writing- Original draft preparation, Resources, Data curation, investigation, Conceptualization, Project administration, Writing - Review & Editing
Acknowledgements: Not Applicable