Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
Gian Maria Pacifici
Associate Professor of Pharmacology via Sant’Andrea 32, 56127 Pisa, Italy.
*Corresponding author: Gian Maria Pacifici, Pharmacology via Sant’Andrea 32, 56127 Pisa, Italy.
Received Date: May 16, 2022
Accepted Date: June 02, 2022
Published Date: June 14, 2022
Citation: Gian Maria Pacifici (2022) “Clinical pharmacology of imipenem”. J Pharmacy and Drug Innovations, 3(4); DOI: http;//doi.org/03.2022/1.1050.
Copyright: © 2022 Gian Maria Pacifici. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Imipenem is a β-lactam antibiotic and is market in combination with cilastatin a drug that inhibits the degradation of imipenem by renal tubular dipeptidase. Imipenem binds to penicillin-binding protein, disrupts bacterial cell wall, and causes depth of susceptible organisms. Imipenem is resistant to hydrolysis by most β-lactamases and is active against a wide variety of aerobic and anaerobic organisms. Imipenem/cilastatin effectively treats urinary-tract, lower respiratory, intraabdominal, gynaecologic, skin, soft tissue, bone, and joint infections. The efficacy and safety of imipenem/cilastatin have been reported and imipenem diffuses into the muscle and subcutaneous tissue in significant amounts. The pharmacokinetics of imipenem have been studied in heathy volunteers and the elimination half-life of imipenem is about 1 hour following the administration of 0.5 grams of imipenem and 2.42 hours following the administration of 1 gram of imipenem. The elimination half-life of imipenem and cilastatin is about 2.5 and 5.4 hours, respectively, in patients with serious renal failure. The treatment and trials with imipenem/cilastatin have been extensively described. Imipenem and cilastatin penetrate into the cerebrospinal fluid in significant amounts and imipenem/cilastatin treats the meningitis caused by Haemophilus influenzae type b, Citrobacter diversus, or Acinetobacter anitratus. Imipenem and cilastatin cross the human placenta in significant amounts and imipenem and cilastatin poorly migrates into the breast-milk. The aim of this study is to review the imipenem/cilastatin efficacy and safety, treatment, trials, treatment of bacterial meningitis, and imipenem and cilastatin pharmacokinetics, transfer across the human placenta and migration into the breast-milk.
Introduction
Antimicrobial activity of imipenem
Imipenem is a β-lactam antibiotic and imipenem is market in combination with cilastatin, a drug that inhibits the degradation of imipenem by a renal tubular dipeptidase. Imipenem binds to penicillin-binding protein, disrupts bacterial cell wall synthesis, and causes death of susceptible microorganisms. Imipenem is very resistant to hydrolysis by most β-lactamases. The activity of imipenem is excellent in-vitro for a wide variety of aerobic and anaerobic microorganisms. Streptococci (including penicillin-resistant Streptococcus pneumoniae); enterococci (excluding Enterococcus faecium and non-β-lactamase-producing penicillin-resistant strains); staphylococci (including penicillinase-producing strains but not methicillin-resistant Staphylococcus aureus); and Listeria (although ampicillin is more active) all are typically susceptible. Activity is excellent against the Enterobacteriaceae with the exception of emerging Klebsiella pneumoniae carbapenemase-producing strains. Most strains of Pseudomonas and Acinetobacter are inhibited, but resistance to imipenem among these organisms is increasing. Anaerobes, including Bacillus fragilis, are highly susceptible. Imipenem also displaces activity against Nocardia species and some species of rapid growing mycobacteria [1].
Therapeutic use of imipenem
Imipenem/cilastatin is effective for a wide variety of infections, including urinary-tract and lower respiratory infections; intraabdominal and gynaecologic infections; and skin, and soft tissue, bone, and joint infections. Imipenem/cilastatin appears to be especially useful for the treatment of infections caused by cephalosporin-resistant nosocomial bacteria. It is prudent to use imipenem for empirical treatment of serious infections in hospitalized patients who have recently received other β-lactam antibiotics. When imipenem is used for treatment of severe Pseudomonas aeruginosa infection, resistant may develop during therapy [1].
Administration, distribution, metabolism, and elimination of imipenem
Imipenem is not absorbed orally. The drug is hydrolysed by a dipeptidase found in the brush border of the proximal tubule. To prolong drug activity, imipenem is combined with cilastatin, an inhibitor of the dehydropeptidase. Both imipenem and cilastatin have an elimination half-life of about 1 hour. When administered concurrently with cilastatin, about 70% of administered imipenem is recovered in the urine as the active drug. Dosage should be modified for patients with renal insufficiency. Nausea and vomiting are the most common adverse-effects (1% to 20%). Seizures have been noted in up to 1.5% of patients, especially when high doses are given to patients with central nervous system lesions and those with renal insufficiency. Patients who are allergic to β-lactam antibiotics may have hypersensitivity reactions when given imipenem, although the incidence of immediate-type hypersensitivity appears to be below 1% [1].
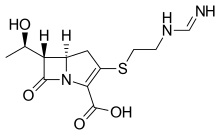
Imipenem molecular structure (molecular weight = 299.347 grams/mole)
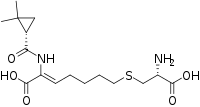
Cilastatin molecular structure (molecular weight = 358.454 grams/mole)
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “imipenem efficacy safety”, “imipenem tissue concentration”, “imipenem pharmacokinetics”, “imipenem treatment”, “imipenem trials”, imipenem CSF”, “imipenem meningitis”, “imipenem placental transfer”, and “imipenem breast-milk”. In addition, the book “The pharmacological basis of therapeutics” [1] has been consulted.
Results
Efficacy and safety of imipenem/cilastatin
Imipenem/cilastatin is efficacy and safe in treatment of patients with complicated intraabdominal infections, hospital-acquired bacterial pneumonia, and ventilator-associated bacterial pneumonia [2]. Imipenem/cilastatin effectively and safety treats severe bacterial infections [3]. Imipenem/cilastatin effectively and safety treats septicaemia [4]. Imipenem/cilastatin is well tolerated and effectively treats septicaemia [5]. Imipenem/cilastatin, administered at a dose of 1 gram 4 times-daily, effectively and safety treats osteomyelitis, bacteraemia, cellulitis, pneumonia, pelvic cellulitis, intraabdominal abscess, empyema, and endometritis [6]. Imipenem/cilastatin effectively and safety treats infections due to gram-positive or gram-negative aerobe and anaerobe bacteria [7]. Imipenem/cilastatin is efficacy and safe in treatment of proven bacterial infections which have failed with other antibiotics [8]. Imipenem/cilastatin effectively and safety treats complicated urinary-tract bacterial infections [9].
Diffusion of imipenem into body-tissues
Imipenem was administered at a dose of 1 gram to patients with serious infection and to healthy volunteers. Imipenem concentration in muscle and in subcutaneous tissue of patients was 2.3+1.5 µg/ml and this concentration was significantly lower than that obtained in healthy subjects which was 12.8+1.6 and 10.7+1.0 µg/ml for muscle and subcutaneous tissue, respectively. The tissue distribution-rate constant for muscle and subcutaneous tissue was 1.95+0.6 and 1.1+0.2 h-1, respectively, in patients, and 5.2+1.0 and 6.6+1.7 h-1, respectively, in healthy subjects. The area under the plasma concentration-time curve was 37.4+5.9 µg*h/ml in patients and 46.0+4.4 µg*h/ml in healthy subjects. The total body clearance was 6.3+0.8 L/h and 13.2+1.4 L/h in patients and healthy subjects, respectively. These data suggest that the pharmacokinetic parameters of imipenem are alternated in patients with serious infection [10].
Pharmacokinetics of imipenem in healthy volunteers
Jaruratanasirikul et al. [11] studied the pharmacokinetics of imipenem in 15 healthy volunteers and imipenem was administered by intravenous infusion at a dose of 0.5 grams or 1 gram 4 times-daily and the infusion lasted 0.5 hours or 2 hours and the pharmacokinetic parameters of imipenem were determined on the third dose of imipenem.
|
|
0.5 hour infusion |
2 hours of infusion |
|
|
|
Dose |
||
|
Parameter |
0.5 grams |
0.5 grams |
1 gram |
|
Peak concentration (µg/ml) |
48.43+5.89 |
21.64+2.22a |
43.91+5.73b |
|
Minimum concentration (µg/ml) |
0.62+0.31 |
1.05+0.45 |
2.27+0.72a,b |
|
AUC0-∞ (µg*h/ml) |
63.71+1.04 |
59.00+6.76 |
127+17.32a,b |
|
Total body clearance (L/h) |
7.95+1.04 |
8.58+1.05 |
8.00+1.12b |
|
Elimination half-life (h) |
1.32+0.27 |
1.02+1.49a |
2.42+0.27a,b |
|
Elimination-rate constant (h-1) |
0.57+0.18 |
0.70+0.17a |
0.29+0.03a,b |
|
Distribution volume (L) |
9.41+1.44 |
9.44+1.76 |
11.60+1.99a,b |
Table 1. Pharmacokinetic parameters of imipenem which have been obtained in 15 healthy volunteers. Values are the mean+SD, by Jaruratanasirikul et al. [11].
AUC = area under the concertation-time curve. aP-value < 0.05 versus 0.5 hours infusion of 0.5 grams of imipenem. bP-value < 0.05 versus 2 hours infusion of 0.5 grams of imipenem.
Pharmacokinetics of imipenem and cilastatin in patients with severe renal failure
Verbist et al. [12] investigated the pharmacokinetics of imipenem and cilastatin in 6 patients with severe renal failure who received imipenem/cilastatin sodium at a dose of 500/500 mg twice-daily. The patients were aged 52.3 years (range, 32 to 66), weighed 64.0 kg (range, 48 to 84), had a body-surface area of 1.69 m2 (range, 1.39 to 2.00), and had a creatinine clearance corrected for body-surface area of 10.4 ml/min1.73 m2 (range 5.34 to 14.8).
|
|
Imipenem |
Cilastatin |
||
|
Parameter |
Day 1 |
Day 5 |
Day 1 |
Day 5 |
|
Peak conc. (µg/ml) |
42.1+12.0 |
43.7+9.8 |
44.6+13.2 |
58.6+10.5 |
|
AUC0-12h (µg*h/ml) |
158+37 |
152+31 |
270+60 |
351+37 |
|
AUC0-∞ (µg*h/ml) |
166+38 |
158+32 |
346+62 |
455+69 |
|
DV (L/kg) |
0.21+0.03 |
0.20+0.03 |
0.17+0.03 |
0.16+0.02 |
|
*T1/2 (h) |
2.75 |
2.49 |
5.45 |
5.24 |
|
TBC (ml/min/1.73 m2) |
57.4+10.2 |
59.0+12.5 |
23.4+3.6 |
21.7+3.4 |
|
UC (ml/min/1.73 m2) |
7.5+3.7 |
6.4+3.0 |
9.8+3.9 |
10.3+4.5 |
Table 2. Pharmacokinetic parameters of imipenem and cilastatin which have been obtained in 6 patients with severe renal failure on the first and fifth day of imipenem/cilastatin sodium administration. Values are the mean+SD, by Verbist et al. [12].
AUC = area under the concentration-time curve. DV = distribution volume. *Elimination half-life (harmonic mean). TBC = total body clearance. UC = urinary clearance.
This table shows that the pharmacokinetic parameter of both imipenem and cilastatin are similar on day 1 and day 5 of administration, the total body clearance is greater than the urinary clearance, and the elimination half-life of imipenem is longer in patients with severe renal failure than in healthy volunteers. For comparison with healthy volunteers see table 1. The comparison of other pharmacokinetic parameters between patients with severe renal failure and healthy volunteers cannot be carried out because the pharmacokinetic parameters are expressed in different units in patients and healthy volunteers.
Treatment of bacterial infection with imipenem/cilastatin
Meropenem/clavulanate is more effective than imipenem/clavulanate in treating multidrug-resistant and extensively drug-resistant patients with tuberculosis [13]. Meropenem is efficacious as imipenem/cilastatin for the empirical treatment of serious bacterial infections [14]. Imipenem/cilastatin treats acute bacterial infections in patients with AIDS and AIDS-related complex [15]. Imipenem/cilastatin treats bacterial infections in burn patients [16]. Cefoperazone/sulbactam is effective as imipenem/cilastatin in treatment of Acinetobacter bacteraemia [17]. Ampicillin/sulbactam treats acinetobacter pneumonia as imipenem/cilastatin [18]. Imipenem/cilastatin effectively treats life-threatening surgical bacterial infections [19]. Imipenem/cilastatin treats lung infections caused by Pseudomonas aeruginosa [20]. Imipenem/cilastatin treats lung bacterial infections in patients with lung cancer [21]. Imipenem/cilastatin treats mild or moderate skin and soft tissue infections and imipenem/cilastatin is well tolerated [22]. Imipenem/cilastatin is a highly effective agent for the treatment of a variety of serious bacterial infections [23]. The intramuscular injection of imipenem/cilastatin is well tolerated and treats bacterial infections [24]. Imipenem/cilastatin treats intraabdominal infections caused by Staphylococcus epidermidis, Morganella morganii, or Fusobacterium varium [25]. Treatment with either ciprofloxacin or imipenem/cilastatin effectively controls severe nosocomial pneumonia [26]. Imipenem/cilastatin effectively treats children with infections caused by Staphylococcus aureus, Streptococcus pyogenes, Haemophilus influenzae, or by Pseudomonas aeruginosa [27]. Imipenem/cilastatin treats children with bronchopneumonia, peritonitis, complicated appendicitis, and septicaemia [28]. Imipenem/cilastatin effectively treats appendicitis in children as does tobramycin plus metronidazole [29]. Imipenem/cilastatin monotherapy treats fever and bacterial infections in neutropenic cancer patients [30]. Imipenem/cilastatin effectively treats bacterial infections in neutropenic cancer patient [31]. Imipenem/cilastatin treats bacterial infections in neutropenic cancer patients [32]. Imipenem/cilastatin sodium is an effective first-line antibiotic for treatment of peritonitis in patients with continuous peritoneal dialysis [33].
Trials with imipenem/cilastatin
Meropenem, administered intravenously at a dose of 500 mg thrice-daily, has comparable safety and efficacy as imipenem/cilastatin given intravenously at a dose of 500/500 thrice-daily [34]. Imipenem/cilastatin, administered at dose of 500/500 mg 4 times-daily, effectively treats severe bacterial infections as meropenem given at a dose of 1 gram thrice-daily [35]. Imipenem/cilastatin has similar efficacy as piperacillin/tazobactam in treatment of pneumonia caused by Pseudomonas aeruginosa [36]. Levofloxacin, administered at a dose of 500 mg twice-daily, is effective and well tolerated as imipenem/cilastatin, given at a dose of 1 gram thrice-daily, in treatment of hospitalized patients with suspected bacteraemia or sepsis [37]. Imipenem/cilastatin has therapeutic efficacy as latamoxef plus tobramycin in treatment of febrile neutropenic patients with lung cancer [38]. Moxalactam is less irritating at the site of injection than imipenem/cilastatin although moxalactam causes more blending episodes [39]. Imipenem/cilastatin is equivalent to ceftriaxone plus gentamicin in treatment of neutropenic sepsis [40]. Imipenem/cilastatin is highly effective in treatment of infection caused by a broad range of gram-positive and gram-negative aerobic and anaerobic bacteria [41].
Penetration of imipenem and cilastatin into the cerebrospinal fluid (CSF)
Jacobs et al. [42] studied the penetration of imipenem and cilastatin into the CSF of 20 children, aged 52+17 months (range, 4 months to 11 years) with meningitis caused by Haemophilus influenzae type B (N = 9), Streptococcus pneumoniae (N = 3), Neisseria meningitidis (N =4), Escherichia coli (N = 1), Staphylococcus aureus (N = 1), or Staphylococcus epidermidis (N = 2) and imipenem/cilastatin was administered by intravenous infusion at a dose of 25 mg/kg thrice-daily (multiple doses) or 25 mg/kg once-daily (single dose). The minimum inhibitory concentration (µg/ml) was: 0.08+0.15 for Haemophilus influenzae, 0.12+0.12 for Streptococcus pneumoniae, 0.06+0.03 for Neisseria meningitidis, 0.25 for Escherichia coli, 0.10 for Staphylococcus aureus, and 1.5+0.7 for Staphylococcus epidermidis.
|
|
Ts (min) |
Serum conc. (µg/ml) |
TCSF (min) |
CSF conc. (µg/ml) |
CSF to serum ratio |
|||||
|
Single dose |
||||||||||
|
|
Early |
Late |
Early |
Late |
Early |
Late |
Early |
Late |
Early |
Late |
|
Mean |
136 |
135 |
8.59 |
9.96 |
148 |
133 |
1.36 |
2.08 |
0.15 |
0.27 |
|
+SEM |
3.5 |
10.3 |
0.95 |
2.3 |
9.6 |
8.0 |
0.32 |
1.4 |
0.03 |
0.15 |
|
Range |
121-160 |
86-175 |
3.4-14.4 |
4.1-23.1 |
118-212 |
105-155 |
0.5-3.7 |
0.3-9.4 |
0.06-0.37 |
0.05-1.12 |
|
N |
9 |
7 |
9 |
7 |
9 |
7 |
9 |
7 |
9 |
9 |
|
Multiple doses |
||||||||||
|
|
Early |
Late |
Early |
Late |
Early |
Late |
Early |
Late |
Early |
Late |
|
Mean |
125 |
150* |
11.97 |
9.57 |
102 |
147 |
1.87 |
1.22 |
0.22 |
0.17 |
|
+SEM |
6.5 |
8.8 |
2.03 |
1.76 |
9.2 |
11.2 |
0.29 |
0.11 |
0.05 |
0.04 |
|
Range |
103-165 |
110-183 |
3.8-25.0 |
2.9-19.2 |
92-185 |
105-214 |
0.27-3.5 |
0.7-1.8 |
0.01-0.31 |
0.07-0.42 |
|
N |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
Table 3: Imipenem concentration in serum and in the cerebrospinal fluid (CSF). Values are the mean, standard error of the mean, range, and number of children, by Jacobs et al. [42].
Ts = time from end of drug administration to serum sampling time. TCSF = time from end of drug administration to CSF sampling time. *P-value < 0.05 for Ts “Late” single dose versus multiple doses. N = number of children. Early = treatment during the first 3 days of therapy. Late = treatment during the last 3 days of therapy.
This table shows that parameters are not different between single dose and multiple doses (except for the late time from end of drug administration to serum sampling time single dose versus multiple doses), the mean time of imipenem administration to serum sampling time is about 130 min, the mean time from end of imipenem administration of CSF sampling time ranges from 102 to 148 min, the mean imipenem concentration is lower in CSF than in serum, the mean CSF to serum ratio of imipenem is 0.27 for the single dose and 0.17 for the multiple doses, and there is a remarkable interindividual variability of the various parameters. This variability is accounted by the wide difference of child ages and child diseases. In addition, imipenem concentration in the CSF is higher than minimum inhibitory concentration of the organisms causing the meningitis.
Table 4. Cilastatin concentration in serum and in the cerebrospinal fluid (CSF). Values are the mean, standard error of the mean, range, and number of children, by Jacobs et al. [42].
|
|
Ts (min) |
Serum conc. (µg/ml) |
TCSF (min) |
CSF conc. (µg/ml) |
CSF to serum ratio |
|||||
|
|
Early |
Late |
Early |
Late |
Early |
Late |
Early |
Late |
Early |
Late |
|
Single dose |
||||||||||
|
Mean |
136 |
135 |
7.41 |
5.73 |
145 |
133 |
1.10 |
1.70 |
0.16 |
0.66* |
|
+SEM |
3.5 |
10.3 |
1.56 |
1.45 |
9.6 |
8.0 |
0.24 |
1.01 |
0.04 |
0.22 |
|
Range |
121-160 |
86-175 |
4.0-16.8 |
1.2-13.3 |
118-212 |
105-160 |
0.5-2.6 |
0.5-6.2 |
0.06-0.35 |
0.30-1.14 |
|
N |
9 |
7 |
7 |
7 |
9 |
7 |
8 |
5 |
6 |
4 |
|
Multiple doses |
||||||||||
|
|
Early |
Late |
Early |
Late |
Early |
Late |
Early |
Late |
Early |
Late |
|
Mean |
124 |
150 |
9.50 |
7.61 |
120 |
147 |
1.59 |
1.04** |
0.29 |
0.21*** |
|
+SEM |
6.6 |
8.8 |
2.33 |
1.87 |
9.2 |
11.2 |
0.21 |
0.12 |
0.08 |
0.07 |
|
Range |
103-165 |
110-183 |
1.6-24.0 |
1.5-15.7 |
92-185 |
105-214 |
0.9-2.6 |
0.6-1.7 |
0.06-0.81 |
0.04-0.67 |
|
N |
9 |
9 |
8 |
9 |
9 |
9 |
8 |
9 |
8 |
8 |
Ts = time from end of drug administration to serum sampling time. TCSF = time from end of drug administration to CSF sampling time. Early = treatment during the first 3 days of therapy. Late = treatment during the last 3 days of therapy. *P-value < 0.05 difference for CSF to serum ratio “Early” versus “Late”. **P-value < 0.05 difference for CSF conc. single dose versus multiple doses. ***P-value < 0.05 difference for CSF to serum ratio single dose versus multiple doses. N = number of children.
This table shows that the parameters are not different in the single dose and multiple doses (except for CSF concentration “Late” single dose versus multiple doses and CSF to serum ratio “Late” single dose versus multiple doses). The mean time from end of cilastatin administration to serum sampling time is about 130 min, the mean time from end of cilastatin administration to CSF sampling time is about 130 min, cilastatin concentration is higher in serum than in CSF, and the mean CSF to serum ratio of cilastatin concentration is less than 1. Cilastatin concentration in the CSF is higher than 1 µg/ml indicating that cilastatin penetrates into the CSF in significant amounts. In addition, there is a remarkable interindividual variability of the parameter and this variability is due to the wide variation of child ages and child diseases.
The penetration of imipenem and cilastatin into the CSF was determined in 10 adult patients with bacterial meningitis who received 4 doses of 1 gram imipenem/cilastatin intravenously. The concentrations of imipenem in CSF ranged from 0.5 to 11 µg/ml and that of cilastatin ranged from 1.1 to 10.5 µg/m, thus imipenem and cilastatin penetrate into the CSF in significant amounts [43].
Treatment of bacterial meningitis with imipenem/cilastatin
Imipenem/cilastatin was administered intravenously at a dose of 500/500 mg 4 times-daily to 21 children aged 3 to 48 months with meningitis caused by Haemophilus influenzae type b. The mean cerebrospinal fluid to serum ratio was 0.14 and 0.10 for imipenem and cilastatin, respectively, and the meningitis was cured in all children [44]. Twenty infants and children had the meningitis caused by Citrobacter diversus and were treated with imipenem/cilastatin intravenously at a dose of 1 gram 4 times-daily and the meningitis was cured in all subjects [45]. Ten adult patients had the meningitis caused by Acinetobacter anitratus and were treated with imipenem/cilastatin intravenously at a dose of 1 gram 4 times-daily and the meningitis was cured after 12 days of treatment [46].
Transfer of imipenem and cilastatin across the human placenta
Heikkilä et al. [47] studied the transfer of imipenem across the human placenta in 7 pregnant women with gestation age of 38.7 weeks (range, 37.0 to 41.0) who received a single intravenous dose of 500/500 mg of imipenem/cilastatin.
Table 5. Concentrations of imipenem in the maternal and foetal plasma which have been measured in 7 women at delivery. Values are the minimum, maximum, mean, and +SD, by Heikkilä et al. [47].
|
|
Imipenem concentration (µg/ml) |
Ratio |
||||
|
|
Maternal plasma |
Umbilical vein Plasma |
Umbilical artery plasma |
Amniotic fluid |
Umbilical vein to maternal plasma ratio |
Amniotic fluid to maternal plasma ratio |
|
Minimum |
1.90 |
0.50 |
0.42 |
0.02 |
0.14 |
0.01 |
|
Maximum |
9.20 |
3.2 |
3.20 |
1.20 |
0.52 |
0.13 |
|
Mean |
5.13 |
1.72 |
1.64 |
0.72 |
0.33 |
0.16 |
|
+SD |
2.81 |
1.22 |
1.22 |
0.85 |
0.12 |
0.25 |
This table shows that imipenem crosses the placenta in significant amounts as the mean umbilical vein to maternal plasma ratio is 0.33.
Imipenem/cilastatin was administered intravenously at a dose of 500/500 mg twice-daily to 7 women at delivery and the concentrations of imipenem and cilastatin in the umbilical cord serum were about 70% of those in the maternal serum [48]. Imipenem/cilastatin was administered intravenously at a dose of 500/500 mg twice-daily to 12 pregnant women at delivery. The umbilical cord serum concentration of imipenem was about 70% of that in the maternal serum 30 min after administration. The mean concentration of imipenem in the amniotic fluid was 1 µg/ml 45 min after dosing and became higher than the maternal serum at 90 min after administration and gradually increased afterward [49]. Imipenem/cilastatin was administered intravenously at a dose of 500/500 mg twice-daily to 81 pregnant women at delivery and the imipenem concentration in the umbilical cord serum was about 70% of that of the maternal serum [50].
Migration of imipenem and cilastatin into the breast-milk
Imipenem/cilastatin was administered intravenously at a dose of 500/500 mg twice-daily to 81 lactating women and the concentration of imipenem in the breast-milk was less than 1 µg/ml [50]. Imipenem/cilastatin was administered intravenously at a dose of 500/500 mg twice-daily to 10 lactating women. Imipenem concentration in the breast-milk was 0.21 and 0.52 µg/ml 1 and 5 hours, respectively, after administration, in one breast-milk sample imipenem was undetectable, and the concentration of cilastatin was undetectable in all breast-milk samples [51]. Imipenem/cilastatin was administered intravenously at a dose of 500/500 mg twice-daily to 11 lactating women and the imipenem concentration in the breast-milk was less than 1 µg/ml at all times up to 6 hours after the dose and the concentration of cilastatin was undetectable in all breast-milk samples [52].
Discussion
Imipenem is a β-lactam antibiotic and imipenem is market in combination with cilastatin a drug that inhibits the degradation of imipenem by renal tubular dipeptidase. Imipenem binds to penicillin-binding protein, disrupts bacterial cell wall synthesis, and causes death of susceptible organisms. Imipenem is active against streptococci, enterococci, staphylococci, Listeria, Enterobacteriaceae, Pseudomonas, Acinetobacter, Bacillus fragilis, and Nocardia species. Imipenem/cilastatin is effective for treatment of urinary-tract, lower respiratory, intraabdominal, gynaecologic, skin, soft tissue, bone and joint infections. Imipenem is not absorbed orally and is administered intravenously. The efficacy and safety of imipenem/cilastatin have been reported [2-9] and imipenem diffuses into muscle and subcutaneous tissue in significant amounts but imipenem concentration in these tissues is lower in patients with serious infection than in healthy volunteers [10]. The pharmacokinetics of imipenem have been studied in healthy volunteers and the mean elimination half-life of imipenem is about 1 hour following the administration of imipenem at a dose of 0.5 grams and is 2.42 hours after the administration of 1 gram of imipenem. In patients with severe renal failure, the elimination half-life of imipenem and cilastatin is about 2.5 and 5.2 hours, respectively. Imipenem is eliminated by renal route and the elimination half-life of imipenem is longer in patients with severe renal failure than in healthy volunteers [12]. The treatment of bacterial infections with imipenem/cilastatin has been extensively studied [13-33] and trials with imipenem/cilastatin have been reported [34-41]. Imipenem and cilastatin penetrate into the cerebrospinal fluid in significant amounts [42-43] and imipenem/cilastatin treats the meningitis caused by Haemophilus influenzae type b [44], by Citrobacter diversus [45], and by Acinetobacter anitratus [46]. The transfer of imipenem and cilastatin across the human placenta is documented by four studies and imipenem and cilastatin are transferred across the human placenta in significant amounts [47-50]. The migration of imipenem and cilastatin into the beast-milk has been described in three studies and both imipenem and cilastatin poorly migrates into the breast-milk [50-52].
In conclusion, imipenem is a β-lactam antibiotic and is market in combination with cilastatin a drug that inhibits the degradation of imipenem by a renal tubular dipeptidase. Imipenem binds to penicillin-binding protein, disrupt bacterial cell wall, and causes death of susceptible organisms. Imipenem is active against a wide variety of aerobic and anaerobic organisms. Imipenem/cilastatin effectively treats urinary-tract, lower respiratory, intraabdominal, gynaecologic, skin, soft tissue, bone, and joint infections. Imipenem is not absorbed orally and is administered intravenously. The efficacy and safety of imipenem/cilastatin have been reported. Imipenem diffuses into muscle and subcutaneous tissue in significant amounts but imipenem concentration in these tissues is lower in patients with infection than in healthy volunteers. Imipenem mean elimination half-life is about 1 hour in healthy subjects and about 2.5 hours in patients with severe renal failure. The treatment and trials with imipenem/cilastatin have been reported. Imipenem and cilastatin penetrates into the cerebrospinal fluid in significant amounts and imipenem/cilastatin treats bacterial meningitis. Imipenem and cilastatin cross the human placenta in significant amounts and poorly migrate into the breast-milk. The aim of this study is to review the clinical pharmacology of imipenem.
Conflict of interests
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.