Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
Faezesadat Heidari1, Habibollah Afshang2, Mohsen Zabihi3*, Ahmad Rafiei kharanagh4, Mandana Mirhosseini5
1Department of Infectious Disease, School of Medicine,Shahid Sadoughi University of Medical Sciences and Health Services, Yazd
2Student Research Committee, Faculty of Pharmacy, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3Department of Pharmacology, Faculty of Pharmacy, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
4Master of laboratory science, Bahar nursing home, Yazd, Iran
5MD, Bahar nursing home, Yazd, Iran
*Corresponding author: Mohsen Zabihi, Department of Pharmacology, Faculty of Pharmacy, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Received Date: July 22, 2023
Accepted Date: August 02, 2023
Published Date: August 08, 2023
Citation: Faezesadat Heidari, Habibollah Afshang, Mohsen Zabihi, Ahmad Rafiei kharanagh, Mandana Mirhosseini (2023) “Early Treatment of Elderly Covid-19 Patients by using Hydroxychloroquine and Interferon Beta 1a”. J Pharmacy and Drug Innovations, 4(1); DOI:http;//doi.org/05.2023/1.1058.
Copyright: © 2023 Mohsen Zabihi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objective: hydroxychloroquine (HCQ) and interferon beta 1a (IF1) were among suggested treatment plans in patients with covid-19 with controversial results. The present study aims to evaluate the role of the abovementioned treatment agents in patients with covid-19 infection resident in an elderly house in Iran.
Method: In this prospective case series study, 87 patients with confirmed covid-19 infection were included. For the patients, HCQ (200 mg three times a day for 10 days) was prescribed. Clinical and laboratory outcomes including postoperative symptoms, vital signs, and treatment prognosis (death or discharge after two weeks was taken).
Results: 87 patients (23 men and 64 women, with a mean age of 71.36 ± 11.38 years) were included in the study. Three patients have been admitted to the Intensive Care Unit center which two of them were expired. The most common side effect of a combination drug was myalgia followed by headache.
Conclusion: Using HCQ in combination with IF1-beta acquired lower clinical manifestations in contrast to previous drug agents So they can be investigated in further clinical trial phases.
Covid-19; hydroxychloroquine; interferon 1beta; treatment prognosis
Introduction:
Covid-19 is a high-concern pandemic with a high transmission rate throughout the world and until now there was no approved treatment plan to encounter the disease (1). Some studies were evaluated the role of Hydroxychloroquine (HCQ) against viral agents as in-vitro studies and the results showed that the abovementioned one, inhibits viral replication (2-5). Whereas in the clinical phase the efficacy of HCQ was controversial (6-9). A randomized clinical trial conducted on 67 Chinese patients showed a significant decrease in body temperature, cough, and recovery time following 400 mg HCQ prescription per day for 5 days (7). The reduction in postoperative symptoms was dose-dependent in another clinical trial (9, 10). Whereas other studies showed no significant difference in clinical outcomes including treatment prognosis after HCQ treatment with different doses (11, 12).
As the abovementioned studies exhibited controversial results, in this case series study we aimed to evaluate and compare the role of HCQ with IF1beta in patients resident in an elderly house in Iran.
Method:
This study was conducted on patients resident in an elderly house in Yazd, Iran from April to June of 2021. After one death report of the elderly home inhabitants on 10 April 2021. The clinical findings suggested that the expired case was infected with COVID-19 and so the laboratory check-up for the other inhabitants started. All patients were COVID-19 positive according to the RT-PCR test from the nasopharyngeal sample (13). The results suggested that all cases had infected with covid-19 and so early treatment with a combination of HCQ and IF1-Beta was started. 200 mg HCQ three times a day for 10 days was prescribed. Prescription in all participants has been conducted whether the patients had symptoms or not. Patients with severe clinical stage were transferred to the Intensive Care Unit. Demographic data including age and gender, and also laboratory data including vital signs (blood pressure and arterial oxygen level and heartbeat), clinical symptoms including cough, headache, myalgia, vomit, diarrhea, conjunctivitis, rhinorrhea, respiratory distress, sore throat along with systemic disease including diabetes mellitus, asthma were evaluated. The need for Vitamin C, D, Favibavir, Corticosteroids also was evaluated. The treatment prognosis including the need to transfer to the Intensive Care Unit and death were evaluated. All measurements were taken two weeks after administration. we prescribed HOQ to patients every 12 hours for 5 days. In total, we used 10 or one blisters for each patient. And we prescribed prednisolone and vitamin C to some patients, and others did not take these drugs.
Statistical analysis was conducted using IBM SPSS (IBM, IL, Chicago) Version 24.0. The data were reported as frequency and in percent. Chi-square and Exact fisher test were conducted to analyze the correlation between the type of treatment agent and clinical outcomes. The significance was considered as p < 0.05.
Results:
87 patients (23 men and 64 women, with a mean age of 71.36 ± 11.38 years) were included in the study. Seven patients (8.04%) had diabetes mellitus, 14 patients (13.7 %) had hypertension. The prevalence of side effects following injection of HCQ and IB1-beta combination was as such: 28 patients (32.2 %) had a headache. 20 patients (23.0 %) exhibited cough, 15 ones (17.2 %) pharyngeal swab, 34 (39.1 %) myalgia, 10 ones (11.5 %) vomitus, 9 patients (10.3 %) diarrhea, 8 (9.3 %) conjunctivitis, 25 (28.7 %) rhinorrhea, 8 (9.3 %) respiratory distress and 15 (17.2 %) showed sore throat.
The mean and standard deviation of continuous data were illustrated in table 1:
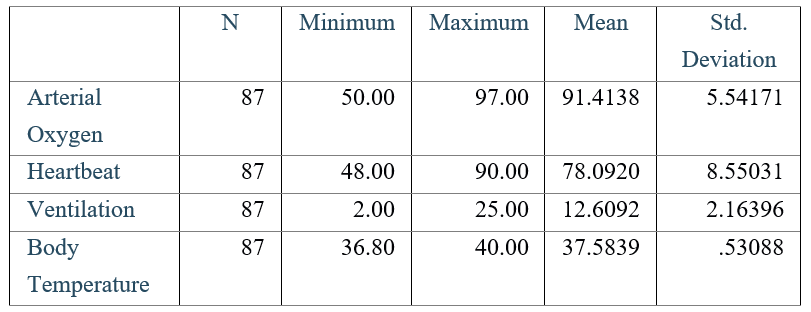
Table 1: The mean and standard deviation of vital signs of patients, two weeks after the follow-up
|
Findings |
N |
(%) |
|
Signs and symptoms Headache Cough Swab Myalgia Vomits Diarrhea Conjunctivitis Rhinorrhea Sore Throat |
28 20 15 34 10 9 8 25 15 |
32.2 23.0 17.2 39.2 14.6 13.0 9.2 28.7 17.2 |
|
Systemic Disease Diabetes Mellitus Hypertension Cardiac Disease Asthma COPD |
20 35 11 3 11 |
23.8 40.2 12.6 3.4 12.6 |
Table 2: Most common clinical findings in patients
Seven patients have been administrated to the Intensive Care Unit which three of them expired. All three were male gender and two of them had COPD disease. This Four patients exhibited some allergic reactions to the drugs including rash and anaphylaxis.
Discussion:
The clinical efficacy of HCQ and IF1-beta combination is controversial in clinical studies. More evidence is needed to confirm its clinical efficacy. In this case serieswe investigated 87 elderly patients affected by COVID-19 in a elderly center of Iran. the follow-up duration was two weeks. most of the patients had mild clinical condition during admission. In this condition, the clinical condition doesn’t get worsen as only seven patients were admitted to the Intensive Care Unit and only three of them were dead. Also, most of the patients were well-tolerated to drug side effects (14) and the most clinical presentation was postoperative myalgia (39.1%) and headache (32.2 %).
In a study conducted by Rahnami et al (14), 80 patients participated in a randomized clinical trial study, the results suggested that injection of IF1-Beta enhanced the rate of participants discharge significantly. The results of another systematic review study revealed that IF1-Beta injection, enhanced discharge level effectively (15). In this study, the combination of HCQ with IF1-beta lowered the mortality rate effectively.
Despite our findings, our study had limitations, incomplete data in some patients make our results analysis more challenging, also unrestricted inclusion criteria, make our study findings less applicable.
In conclusion, based on our findings and its comparison with other studies, it has been confirmed that Hydroxychloroquine and Interfrone-1beta can be used as a combination drug agent since they have low mortality rate and postoperative side effects, although their clinical implication needs more evidence in cases of randomized clinical trials and larger samples.