Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
Sunil Chaudhry
Honorary Medical Director, Bioclinitech Technologies Pvt Ltd, Mumbai, India & GPATtutor.com
*Corresponding author: Sunil Chaudhry, Honorary Medical Director, Bioclinitech Technologies Pvt Ltd, Mumbai, India & GPATtutor.com.
Received: November 15, 2021
Accepted: December 03, 2021
Published: December 07, 2021
Citation: Sunil Chaudhry (2021) “Novel Pharmacotherapeutics for managing Multiple Myeloma”. J Pharmacy and Drug Innovations, 3(1); DOI: http;//doi.org/03.2020/1.1040.
Copyright: © 2021 Sunil Chaudhry. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The median survival of patients with newly diagnosed MM was approximately 2.5 years, before introduction of Proteasome inhibitors and immunomodulatory drugs . Bortezomib, thalidomide, and lenalidomide, and the introduction of autologous stem cell transplantation (ASCT) have substantially improved overall survival (OS), which currently ranges from 5 to 7 years. Several three-drug (triplet) combination regimens have shown efficacy in multiple myeloma. Currently, many MM cell antigens are targeted, and vaccines using whole or partial protein sequences are combined with autologous stem cell transplantation and lymphocyte infusion for treatment. Treatment of Multiple myeloma cost in country is about 12000 USD.
Introduction: Multiple myeloma (MM) is a plasma cell dyscrasia characterized by monoclonal plasma cells proliferation in bone marrow, resulting in an overabundance of monoclonal paraprotein (M protein), destruction of bone, and displacement of other hematopoietic cell lines. [1]. In India prevalence is in 1.4/1,00,000 population with incidence of 6000 new cases/year. [2]. The overall 5-year survival rate for people with multiple myeloma is 54%. Survival rates though have steadily increased over time. Risk factors of multiple myeloma include age > 65 years, male , family history , over weight or obese , radiation exposure , contact with chemicals [ 3] . Common symptoms may include, bone pain, often in the back or ribs, fractures , weakness or fatigue, weight loss, frequent infections and fevers, feeling thirsty and frequent urination. The diagnostic criteria for multiple myeloma require confirmation of (a) one major criterion and one minor criterion or (b) three minor criteria in an individual who has signs or symptoms of multiple myeloma.
|
Major Criteria |
Minor Criteria |
|
Plasmacytoma |
10% to 30% plasma cells in a bone marrow sample |
|
>30% plasma cells in a bone marrow sample
|
Osteolytic lesions |
|
Elevated levels of M protein in the blood or urine
|
Minor elevations in the level of M protein in the blood or urine
|
Table 1: Types of Diagnostic Criteria
In healthy bone marrow, < 5% of the cells are plasma cells, whereas in people with MM, > 10 % of the cells may be plasma cells [4].
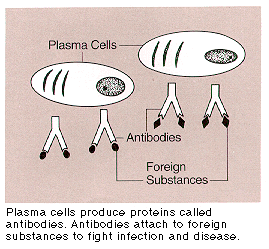
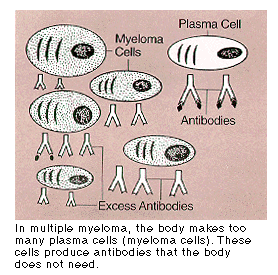
Figure 1: Pathogenesis of Multiple myeloma
Radiographic features : Numerous, well-circumscribed, lytic bone lesions , punched out lucencies and raindrop skull , endosteal scalloping can be visualized. Vertebral compression fractures, long bone fractures are also seen. The predominant areas of bone disease involvement are the axial skeleton, including the vertebral bodies (49%), skull (35%), pelvis (34%), and ribs (33%), and the proximal metaphysis of long bones, especially the femur and humerus [5]
Staging of Multiple myeloma: Early : Asymptomatic: This means the person does not have symptoms and signs of the disease. Late: Symptomatic Calcium levels are increased, which is known as hypercalcemia. Calcium level greater than 0.25 mmol/L. Renal, or kidney, problems, identified as a creatinine level higher than 173 mmol/L. Hemoglobin level that is less than 10 g/dl and Bone lesions( refer as CRAB). [6]
|
MGUS = monoclonal gammopathy of undetermined significance.
|
SMM = smouldering multiple myeloma.
|
MM = multiple myeloma
|
|
M-protein <30 g/L in the serum
|
Serum M-protein ≥30 g/L, and/or urine M-protein ≥500 mg/24 h, and/or bone marrow clonal plasmacytosis 10 - 60%
|
Clonal bone marrow plasmacytosis ≥10% or biopsy-proven plasmacytoma
|
|
<10% clonal plasma cells in the bone marrow Absence of myeloma-defining events or amyloid
|
Absence of myeloma-defining events or amyloid
|
Bone lesions: ≥1 osteolytic lesion(s) on skeletal survey >1 focal lesion on MRI >1 focal lesion on MRI
|
Table 2: Progressive stages of Multiple myeloma
[7]
Drugs for managing Multiple myeloma:
|
Drug Class |
Examples |
|
Proteasome Inhibitors |
Bortezomib, Carfilzomib, Ixazomib |
|
Immunomodulatory drugs |
Thalidomide, Lenalidomide, Pomalidomide |
|
Histone deacetylase inhibitors |
Panobinostat |
|
Antitumor antibiotics |
Doxorubicin |
|
Alkylating agents |
Melphelan, Cyclophosphamide, Bendamustine |
|
Monoclonal antibodies |
Daratumumab Elotuzumab |
Table 3 : Classification of Drugs
[8]
|
Proteasome inhibitor |
Chemical class |
Binding Kinetics |
Half Life in minutes |
Maximal proteasome inhibition at Maximum tolerated dose (%) |
|
Bortezomib |
Boronate IV or SC |
Reversible |
110 |
65-75 |
|
Carfilzomib |
E;poxyketone IV |
Irreversible |
<30 |
>80 |
|
Ixazomib |
Boronate oral |
Reversible |
18 |
73-99 |
|
Oprozomib |
Epoxyketone Oral |
Irreversible |
30-90 |
>80 |
|
Marizomib |
B – Lactone Oral or IV |
Irreversible |
10-15 |
100 |
Table 4 : Classification of Proteasomes:
[9]
Bortezomib is a dipeptide boronic acid derivative and proteasome inhibitor used to treat multiple myeloma and mantle cell lymphoma. The 26S proteasome is a protein complex that degrades ubiquitinated proteins in the ubiquitin-proteasome pathway: reversible inhibition of the 26S proteasome, leading to cell cycle arrest and apoptosis of cancer cells, is thought to be the main mechanism of action of bortezomib. Proteasomes are large protein complexes inside cells they degrade misfolded proteins. Proteasome inhibitors are drugs that block the action of proteasomes cellular complexes that break down proteins.
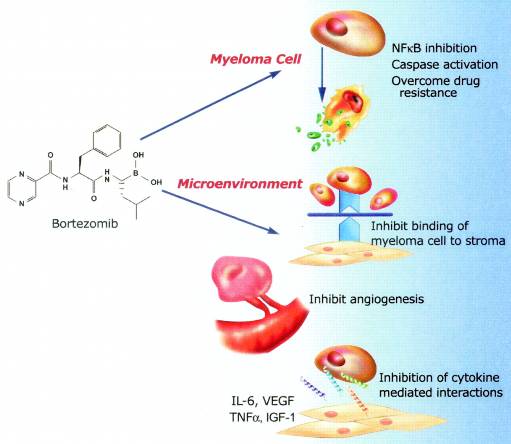
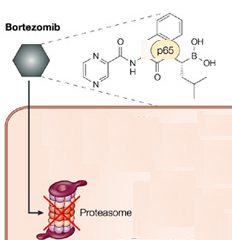
Figure 2: Mode of action of Bortezomib
The mechanism of action of bortezomib involves stabilization of NF-κB, p21, p27, p53, Bid, and Bax, inhibition of caveolin-1 activation, and activation of JNK as well as the endoplasmic reticulum stress response. [10] Subcutaneous administration of bortezomib appears to be comparable with intravenously administered bortezomib in terms of overall systemic availability and response rates in multiple myeloma, but may have an improved safety profile, with fewer dose reductions and discontinuations due to adverse events. Peripheral neuropathy and thrombocytopenia are the key dose-limiting toxicities of bortezomib-based combination regimens. Carfilzomib has demonstrated activity against bortezomib-resistant cell lines and primary multiple myeloma (MM) cells [11]
Immunomodulatory drugs : IMiDs are known to suppress the production of tumor necrosis factor-alpha (TNF-α), interleukin-12 (IL-12), IL-16, IL-1β, macrophage inflammatory protein-1 alpha (MIP-1α and monocyte chemoattractant protein-1 (MCP-1), while increasing the yield of IL-2, IFN-γ, IL-10 . Acts on NK cells directly or indirectly. As such, IMiDs , enhances NK-cell cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) in MM cells. These drugs also downregulate tumor necrosis factor also appears to augment NK cell activity via a variety of mechanisms. The treatment of MM cells with lenalidomide significantly increased the expression of b-catenin , suggesting that b-catenin might be an IMiDs’ target protein’s indirect downstream factor. These are promising anti-myeloma agent that has the ability to induce remission in patients with heavily pre-treated MM. Lenalidomide can cause significant neutropenia and thrombocytopenia , pulmonary embolism (PE) in patients with multiple myeloma
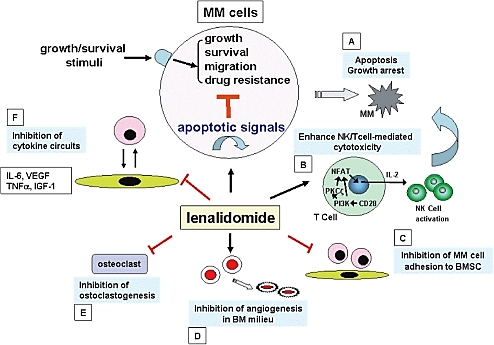
Figure 3: Mode of action of IMIDs
[12]
Panobinostat is a potent oral nonselective pan-histone deacetylase inhibitor (HDAC). It has been approved by the FDA and EMA in combination with bortezomib and dexamethasone for the treatment of multiple myeloma, in patients who have received at least two prior regimens, including bortezomib and an immunomodulatory agent.[13] . Panobinostat is an inhibitor of all class I (HDACs 1, 2, 3, and 8), class II (HDACs 4, 5, 6, 7, 9, and 10), and class IV (HDAC 11) HDACs, with half maximal inhibitory concentrations in the nanomolar range.
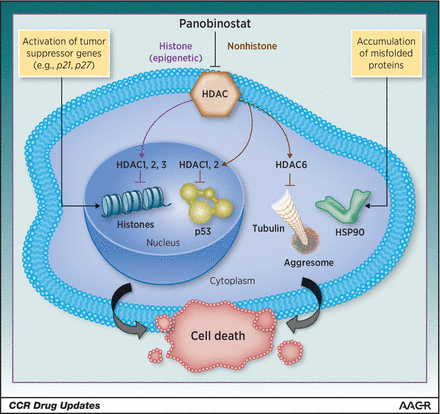
Figure 4: Mode of action of Panobinostat
Panobinostat produces dose related grade 3/4 adverse events (AE) included thrombocytopenia (42%), fatigue (21%), and neutropenia (21%). The most common dose-limiting toxicities included thrombocytopenia, fatigue, and cardiac-related events .[14]
Doxorubicin : is used in combination with (bortezomib) for the treatment of patients with multiple myeloma who have not previously received bortezomib and have received at least one prior therapy. Doxorubicin inhibits the enzyme topoisomerase II, causing DNA damage and induction of apoptosis. The liposomal formulation of doxorubicin has FDA approval for the treatment of ovarian cancer in patients who have failed platinum-based chemotherapy, AIDS-related Kaposi sarcoma, and multiple myeloma
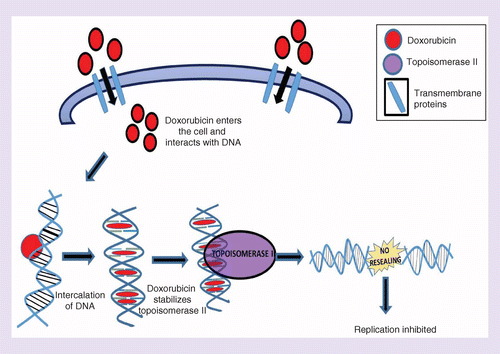
Figure 4 : Action of Doxorubicin
fatigue, alopecia, nausea and vomiting, and oral sores and bone marrow suppression are common side effects. [15]
Alkylating Agents : Bendamustine has proven activity in both newly diagnosed and relapsed-refractory MM. Bendamustine has also demonstrated activity in MM after relapse from ASCT ( Autologous stem cell transplant ) and has recently been used successfully as a conditioning regimen for ASCT in combination with melphalan . PFS and the OR rate were at least 2-fold greater with bendamustine than with chlorambucil [16]
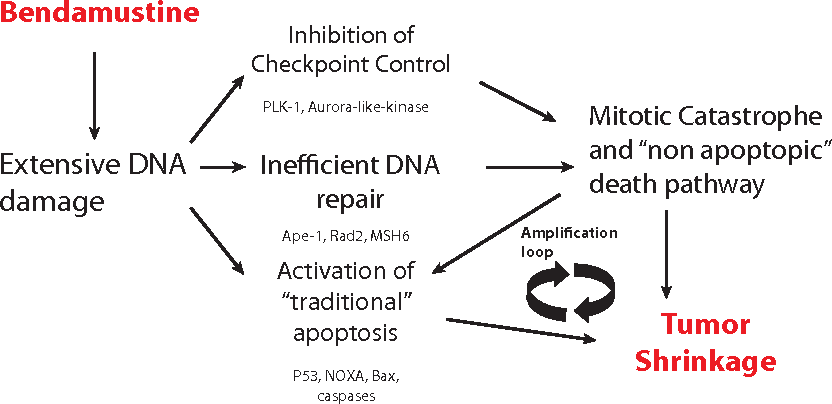
Figure 5 : Mode of action of Bendamustine
The antineoplastic effect of bendamustine is due to production of both single- and double-strand breaks The DNA breaks, observed after bendamustine exposure, are more extensive and significantly more durable than those induced by other alkylators. Side effects reported are fever, chills, or itching during or shortly after the injection; low white cell count are reported . signs of tumor cell breakdown such as confusion, weakness, muscle cramps, nausea, vomiting, bradycardia or tachycardia , decreased urination are also observed.
Melphalan, Cyclophosphamide, and Dexamethasone (MCD) as a salvage regimen for heavily treated relapsed or refractory multiple myeloma patients [17]
Daratumumab: is an IgG1κ fully human mAb that targets CD38, a type II transmembrane glycoprotein composed of extracellular, transmembrane, and intracellular domains. Direct effects are mediated by inhibition of intracellular signal transduction, and by inhibition of CD38 enzymatic activity, which leads to decreased levels of immunosuppressive adenosine. Fc-dependent mechanisms include complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), Daratumumab has shown encouraging results both as monotherapy and in combination with other regimens in both R/R MM and untreated disease. [18]
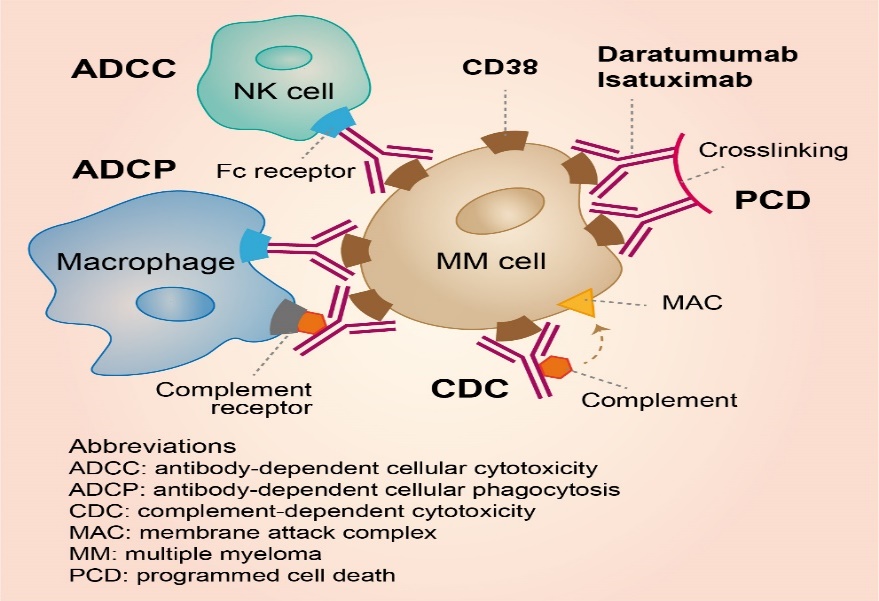
Figure 6 : Daratumumab action
Elotuzumab is a humanized monoclonal antibody used in relapsed multiple myeloma. Elotuzumab directly activates Natural Killer cells through both the SLAMF7 pathway and Fc receptors. Elotuzumab also targets SLAMF7 (Surface antigen CD319 (SLAMF7) is a robust marker of normal plasma cells and malignant plasma cells in multiple myeloma.) and facilitates the interaction with Natural Killer cells to mediate the killing of myeloma cells through antibody-dependent cellular cytotoxicity (ADCC). Useful in relapsed / refractory MM. Elotuzumab , pomalidomide and dexamethasone combination is used in refractory cases. Fatigue, diarrhoea , constipation and cough are commonly observed. [19]
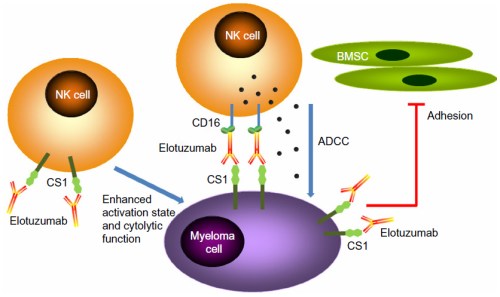
Figure 7: Elotuzumab action
Steroids are commonly used for multiple myeloma treatment and are used at all stages of the disease. In high doses, steroids can lyse multiple myeloma cells. Dexamethasone and the other steroids are useful in myeloma treatment because they can stop white blood cells from traveling to areas where cancerous myeloma cells are causing damage. This decreases the amount of swelling or inflammation in those areas and relieves associated pain and pressure.[20]
Drug combinations in Multiple myeloma :
[21]
|
Trials |
MM03 |
Endeavour |
Aspire |
Tourmaline |
Castor |
Pollux |
Eloquent 1 |
|
Overall Response Rate % |
31 vs 10 |
77 vs 63 |
87 vs 66 |
78 vs 71 |
83 vs 63 |
93 vs 76 |
79 vs66 |
|
Median Progression free survival |
3.8 vs 1.9 |
18.7 vs 9.4 |
26.3 vs 17.6 |
20.6 vs 14.7 |
NR vs 7.2 |
NA |
19.4 vs 14.9 |
|
Grade 3/ 4 Adverse events |
|
|
|
|
|
|
|
|
Haematological |
Neutropenia 48 % |
Thrombocytopenia 8 % |
Anemia 18% |
Neutropenia 22 % |
Lymphocytopenia 10 % |
Anemia 8 % |
Anemia 19 % |
|
Non Haematological |
Fatigue 5 % |
Hypertension 9 % |
Hypertension 4 % |
Diarrhoea 6 % |
Fatigue 6%
|
Hypertension 7 % |
Fatigue 8 % |
|
|
Pramalidomide + DXM vs High dose DXM |
Carfilzomib + DXM versus Bortezomib + DXM |
Carfilzomib + Lenalidomide + DXM vs Lenalidiomide + DXM |
Ixazomib + Lenalidomide + DXM
Vs Lenalidomide + DXM |
Daratumumab + Bortezomib + Dexamethasone vs Bortezomib + Dexamethasone |
Daratumumab + Lenalidomide + Dexamethasone vs Lanalidomide + Dexamethasonme |
Elotuzumab + Lenalidomide + Dexamethasone vs Lenalidomide + Dexamethasone |
Table V : Comparative Trials on Multiple myeloma
Drugs under evaluation: Many therapeutic drugs are under evaluation for multiple myeloma, mostly in combination with dexamethasone and Proteasome inhibitors
|
Drug class |
Types |
Mode |
Studies |
|
mTOR inhibitors |
Everolimus Temsirolimus |
Regulation of cell growth, protein synthesis and cell progression |
Phase 1/2, in combination with bortezomib or lenalidomide |
|
MEK1/2 inhibitor |
Trametinib |
Inhibition of cell growth |
Phase 1, in combination with afuresertib in solid tumors and MM |
|
BRAF inhibitor |
Vemurafenib |
Inhibition of the constitutionally activated NRAS– |
Retrospective data in combination in Phase II trials |
|
AKT inhibitor |
Afuresertib |
Inhibition of cell growth, apoptosis promotion |
Advanced hematologic malignancies including MM |
|
anti IL-6 |
Siltuximab |
Promoting cell-apoptosis by blocking IL-6 through a chimeric anti-IL6 monoclonal antibody |
randomized, in combination with bortezomib or placebo |
|
Antibody drug conjugates |
Belantamab
Lorvotuzumab Milatuzumab |
Anti-BCMA (B-cell maturation antigen) Anti-CD56 Anti-CD138 |
In August 2020, the FDA granted accelerated approval to belantamab mafodotin-blmf as a monotherapy treatment for relapsed or treatment-refractory multiple myeloma. |
|
Alkyl phospholipid inhibitor |
Perifosine |
Inhibitor of PI3K/Akt and MEK/ERK Pathway
|
phase 3 study of perifosine combined with bortezomib and dexamethasone is effective
|
|
XPO-1 inhibitor |
Selinexor
|
Selinexor is an oral, potent XPO-1 inhibitor |
combination of selinexor, bortezomib and dexamethasone in patients with less advanced disease. This regimen allowed an ORR of 63% |
|
chimeric antigen receptor T-cell therapy |
Orvacabtagene Autoleucel |
a B-cell maturation antigen (BCMA)-directed CAR T cell therapy |
patients (pts) with relapsed/refractory multiple myeloma (RRMM) |
Table VI : Drugs under evaluation
PVX vaccine is a tri-peptide vaccine for multiple myeloma. This vaccine recognizes three different proteins that are present in on multiple myeloma cells.
[22]

Table 7 : Algorithm for treating Multiple myeloma : Frontline Therapy of Symptomatic Multiple myeloma
[ASCT – Autologous Stem Cell Transplant, MPT, melphalan, prednisone, thalidomide; VMP, bortezomib, melphalan, prednisone;
CTD, cyclophosphamide, thalidomide, dexamethasone; MP, melphalan, prednisone; VTD, bortezomib, thalidomide,
dexamethasone; VCD, bortezomib, cyclophosphamide, dexamethasone; PAD, bortezomib, doxorubicin, dexamethasone;
RVD, lenalidomide, bortezomib, dexamethasone, BP, bendamustin, prednisolone]
[23]
Indications of radiation therapy: Palliation of bone pain, spinal cord compression , involvement of cranial nerves . RT can produce definitive cures in solitary plasmacytomas, its role in MM is only palliative.
Conclusion: MM accounts for 1.8% of all malignancies and 10–15% of hematologic malignancies. The historic induction therapy for MM consisted of corticosteroids alone, melphalan/prednisone, or the combination of vincristine, doxorubicin, and dexamethasone (VAD) , with median survival of 2- 3 years. The introduction of immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs), and monoclonal antibodies (MABs) have improved treatment outcomes and extended the median OS 5–15+ years. Daratumumab , Elotuzumab have shown encouraging results both as monotherapy and in combination with other regimens in both R/R MM and untreated disease. ASCT is not curative in MM, but improves median OS by ~12 months. The treatment-related mortality rate is 1–2%. In ~50% of patients, ASCT can be done on an outpatient basis. Renal impairment (RI) is one of the most common complications of symptomatic MM, affecting 20–30% of patients at the time of diagnosis and around 10% of them require haemodialysis, with a negative impact on prognosis.