Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
1,2Maria V. Tejada-Simón, PhD, MEd. and 2Vaneeza Sohail Okorie
1College of Pharmacy University of Houston, Texas USA.
2College of Natural Sciences and Mathematics University of Houston, Houston, Texas, USA.
*Corresponding author: Maria V. Tejada-Simón, Associate Professor of Pharmacology and Director of Faculty Development College of Pharmacy University of Houston 4849 Calhoun Road Houston, Texas 77204, USA.
Received: November 05, 2021
Accepted: November 12, 2021
Published: November 16, 2021
Citation: Maria V. Tejada-Simón, Vaneeza Sohail Okorie (2021). “Update on Drug Repurposing for Neurological and Neuropsychiatric Conditions”. J Pharmacy and Drug Innovations, 3(1); DOI: http;//doi.org/03.2020/1.1038.
Copyright: © 2021 Maria V. Tejada-Simón. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Drug repurposing is an old practice. In recent years, there has been a rise in the number of FDA- approved drugs being studied for effectiveness in other diseases than the one for which they were originally discovered and developed. Repurposed drugs are being utilized widely, to treat pain, neurodegenerative conditions, cancer and other rare diseases for which no drug treatment exists currently. In general, the process of repurposing a drug is usually funded not from pharmaceutical companies, but by separate sectors and organizations which have an interest, depending on the targeted disease, and who will most likely have benefits post-commercialization. This may include non-profit organizations, patient advocacy groups and some government sectors. The drugs must go through a specific procedure to get approval, with the final step being their commercialization. The support of a FDA-approved drug for a different use is dependent on stablished guidelines and other ethical responsibilities. Approval may be facilitated if the observed effects are positive or the disease recovery is significant, especially when involving oncological disorders. Repurposing drugs, when they have observed and reported benefits, is very important, because there is lower cost involved since the FDA previously approved these compounds. Consequently, less time, money and research effort needs to be spent getting approval, and more time can be spent working on clinical trials that demonstrate the effectiveness on an ‘off-label’ condition. In this synopsis, we explore the advantages and disadvantages of drug repurposing, the approaches taken for drug repositioning, as well as a description of some important drugs used to treat neurological and psychiatric disorders that are being considered for other conditions.
Introduction
Drug repurposing, also known as drug repositioning, re-profiling or re-tasking, refers to the re-evaluation of known drugs for new uses or molecular targets different from a drug’s accepted action, pharmacological outcome or binding specificities (Sam and Athri, 2017). It is an ongoing phenomenon involving both the research and clinical fields, both trying to find unknown additional uses for an existing chemical or compound. Theoretically, a drug discovered and used for a certain illness can be used to treat another disease. The most known example of drug repurposing is probably aspirin, which has been around for years. Initially approved as a painkiller, it was later observed that it could prevent cardiovascular problems, thus, its use was expanded to prevent cardiovascular disease (Peters and Mutharasan, 2020).
Drug repurposing is a booming industry based almost entirely in trial and error after certain observed new drug mechanisms and effects. The repurposing of already existing drugs may be beneficial when perceived from an economic point of view. Repurposing happens after a drug or compound has been already approved, reducing then the cost of additional testing and acceptance. The additional work and time is then placed in clinical trials for that particular compound targeting a new condition (Langedijk et al., 2015).
During the additional research and clinical trials, it is crucial to keep patient’s welfare a priority. To evaluate the drug’s effect on a new condition, the trials must be carried out under the supervision of experts, to ensure participants safety. To study a drug for possibly re-tasking we must first:
Pros and Cons of research for Drug Repurposing
Drug repurposing has benefits and complications, especially during the trial phase, when adding a non-approved medication to the regular regimen of participants. It is a risky practice to recommend a treatment plan using ‘off-label’ drug treatment because of the chance of unknown and sporadic side effects on the patient. Trial participants affected by illnesses like diabetes, heart disease or digestive issues, perhaps all of them at once, might already suffer side effects from any of their medications. Throwing a trial medication into the mix can either be beneficial for that participant by relieving symptoms due to serendipity, or cause an increase of side effects and symptoms adding to their existing illnesses (Pushpakom et al., 2019).
When drugs are prescribed, they are prepacked for a specific disease, and recommended to be used for what is intended and not for other purposes. When a patented drug is suspected to have additional benefits, occasionally the companies that produced the drug may choose to withhold them for possible repurposing, or make them exclusively available for short periods of time. The idea is to have time to reformulate the approved patented drug for an unclaimed disease, and commercialize it with a new target. The repurposed drug will then be confidently sold after conducting research and trials to properly tailor it to a new condition (Sam & Athri, 2019; Elder and Tindal, 2020) but that might take long. However, in some cases, the research will point to harmful effects on patients; therefore, it would be ethically irresponsible to continue the support.
To get a repurposed drug approved by the FDA requires less effort, as the drug has been approved beforehand for another target-disease. However, in some cases in which the drug is being considered for a completely different disease, to obtain the approval might be still problematic. Nevertheless, there might be physicians ready to prescribe them, even if they still are ‘off-label’. In these cases, the physician must be extremely careful when explaining the treatment plan in order to avoid possibly legal issues and dangers. Physicians must use consistent and clear terms to avoid any confusion, making sure the patient understands the goal pursued by using that particular drug (Parvathaneni et al., 2020).
Different Approaches for Drug Repurposing
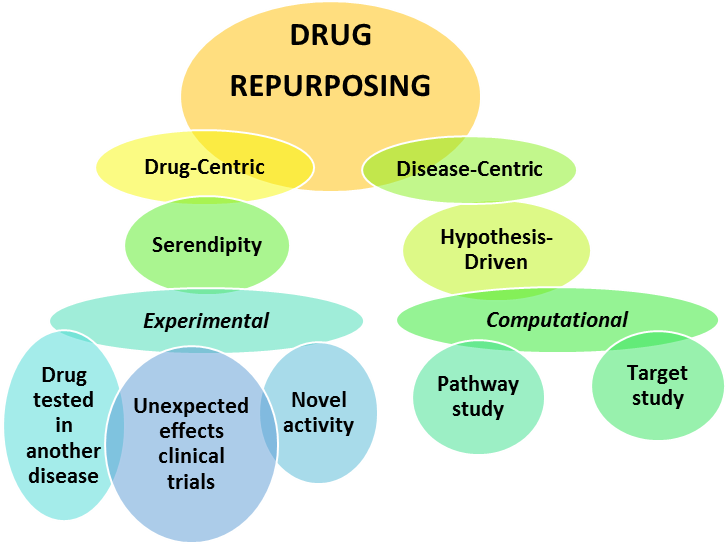 |
Figure 1: Representation of the approaches to Drug Repurposing
There are several approaches towards drug re-tasking. The process can follow a drug-centric model (predicting new indications for an existing drug, using the drug action, target and/or pathway), or a disease-centric approach (which assumes either that many diseases share similarities, or observed unexpected side effects and novel activities) (Figure 1). Drug-centric approach is mostly based on serendipity, while disease-centric approach is more hypothesis-driven.
Both approaches also differ in the way the research is conducted using either the computational or the experimental inquiry (Parvathaneni et al., 2020). The computational research can include target-based methods, knowledge-based conclusions, pathway founded, and signature (regarding the genome) approach. To get the most reliable data showing that repurposing a certain drug can be beneficial, the methods used should find patterns in datasets that use algorithms, systems and models. This approach is complicated and requires a strong computational background (Sam & Athri, 2019). However, there is a need for scientists to study computational methods, because they are the most reliable method to evaluate drug repurposing. Although software has been developed to be consumer friendly and facilitate data understanding, it still requires a strong background in computation. Further advances are needed to make this field of research more accessible to basic scientists without that background, but capable of using web-based tools. With the use of these tools, researchers will have better insight on biological and chemical properties of drugs and advance them to further uses (Masoudi-Sobhanzadeh et al., 2020).Presently, certain web-based software can predict drug-target interactions, linking drug and disease, interpreting gene expression connections (Sam and Athri, 2019), and resulting in databases that are essential when considering drug repositioning.
The experimental research, on the other hand, gives accessibility to life scientists that have deeper understanding into the chemical and biological properties of drugs. It includes two specialized tactics, 1) binding Assays and 2) phenotypic approaches. Binding assays include techniques such as proteomics, a large-scale study of the target proteins in a disease. A recent example is the study of COVID-19 virus, with the effort trying to determine the main proteins involved in its lifecycle and replication, and the consequent identification of approved drugs that could affect those properties (Sultana et al, 2020; Schindell et al., 2020).
When we study the current situation on drug repurposing, it is quite apparent that most of the drug repurposing successes have reached by serendipity. Attempts to reposition certain drugs that produced some success can be seen before 2004, but the most important research and literature is recent (Sam & Athri, 2019; Schindell et al., 2020). In the early 2000’s, there are several pioneers that described biopharmaceutical companies’ attempts to reposition certain drugs for new indications, as a way to increase their productivity/profits and reduce losses from the investment in the study of drugs that did not yield the expected results (Ashburn and Thor, 2004). Since then, drug repurposing articles rose in numbers, reaching more than 600 by 2018, indicating that drug repurposing is a growing industry and has a confident future.
Candidacy for Drug Repurposing
The field of drug repurposing is not limited to one area. People from different sectors, ranging from obstetrics to neurology, are interested in drug repositioning. The cases differ and so does the method and approach for repurposing. For one area, serendipity may be the source of information, while for another there may be thorough research before the repurposed drug is approved and prescribed. Computational approaches are still used, even if serendipity led the research. In the end with trial-and-error, medications are studied, some of them will be successful and others not. In any case, drug repurposing work has become crucial in the development of either ‘new’ drugs, or to treat orphan diseases, with no stablished treatment (Ozery-Flato et al., 2020).
Candidates for repositioning go normally through extended controlled trials in order to reduce bias and obtain approval. However, sometimes other factors alter the process. For instance, recently several randomized controlled trials were conducted searching for a treatment against COVID-19 (Schindell et al., 2020). More than 80 clinical trials took place utilizing computational methods, proposing the use of human immunoglobulin, interferons, chloroquine, hydroxychloroquine, arbidol, remdesivir, favipiravir, lopinavir, ritonavir, oseltamivir, methylprednisolone, bevacizumab, and traditional Chinese medicines (TCM). FDA preapproved all these hence, and a list of ongoing clinical trials was released. Each drug listed was involved in separate clinical trials and in different parts of the world. Some of the medications were used to treat infections, influenza and even malaria. The range was wide and the purposes unlimited, but the hope was that some would work against the new virus. While all these trials were ongoing, one drug from the list went through market sale spikes, Hydroxychloroquine. This was due to the influence of major political statements, which altered the regular research process, driving the prices for that drug up as well as its holdings (Rosa & Santos, 2020).
Several drugs represent the pinnacle of success when it comes to repurposing. For example, Aspirin is a medication that is present in the majority households, and it is used as an analgesic, dispensed for pain. However, in the early 1900’s it was discovered that aspirin could prevent cardiovascular events. Aspirin is now used as a blood thinner, recommended after suffering a first heart attack. Currently, after some research, aspirin might be repurposed again, after discovering that it produces COX-2 inhibition, making it useful to battle colorectal cancer (Jourdan et al., 2020).
Another important drug, Thalidomide, went through a similar path as aspirin. Thalidomide was originally intended as sedative, but soon started being used for the treatment of nausea during pregnancy with terrible consequences (Kim and Scialli, 2011). Soon after that, it was apparent that the drug was useful for treating leprosy and multiple myeloma. Thus, a tinted drug due to the tragedy it produced, ended up being repurposed and helping in this type of cancer (Kim and Scialli, 2011).
In other cases, the condition for which a drug was repurposed resulted in the highest marketing effect for the drug. That is the case for Sildenafil and Minoxidil. Sildenafil (known as Viagra) is a drug that was repurposed before it was even accessible in the market. Its development to reduce hypertension made it very suitable to treat this cardiovascular condition. However, during clinical trials in the United Kingdom, it was observed that one of the side effects was penile erections, what opened up a new purpose for the drug and a great marketing possibility (Beachy et al., 2014). Similarly, Minoxidil was developed as an antihypertensive agent, but in clinical trials, it was observed that the patients taking it started growing more hair, thus the possibility of repurposing that drug as a treatment for alopecia became a reality, profiting for that harmless side effect (Zins, 1988).
Drug repositioning for neurological and psychiatric conditions
Comparable to these key drugs, several neurology related drugs could follow similar steps (Jourdan et al., 2020). However, when dealing with neurological and psychiatric disorders, drug repurposing involves multidimensional issues. All these conditions have the complicated brain chemistry in common.
Parkinson’s Disease
Parkinson’s disease (PD) affects about 200,000 people in the United States of America annually. The symptoms may include tremors, fatigue, constipation and bradykinesia. Other symptoms may be slowness in movement and activity as well as impaired equilibrium and synchronization. Some of the psychiatric related symptoms of PD are apathy, irritability, sadness, insomnia, obsessive-compulsive disorder (OCD), mania and/or bipolar disorder (Boucherie et al., 2021). Other more overlooked symptoms might include behavioral changes such as shorter attention span and decreased school/work performance. The turnover of medication developed to effectively treat PD has been inadequate. Although PD might not have life threatening risks, it still has an adverse effect on the quality of life of people affected by it. The drugs typically used to reduce the effects of PD symptoms include increased levels of Dopamine. The main available drug in the market is Levodopa, also known as L-dopa. Levodopa can be synthesized from Tyrosine, making of this aminoacid a precursor of Dopamine in the system. Levodopa, when administered, may have adverse side effects such as vomiting, nausea and decreased blood pressure. In some patients, it also results in agitation. To reduce these symptoms, Levodopa is usually administered with Carbidopa.
Shortage of dopamine in the nerve cells of the brain is responsible for most symptoms of PD. Several drugs are being considered to be repositioned for treatment of PD. Those are Nilotinib, Inosine and Isradipine (primarily used for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia (Ph+CML, as a supplement and hypertension, respectively). Although there has been a lack of success during the trials conducted since 1999, there is still a spark of hope for those drugs to be somehow effective for PD symptoms. Patients are still willing to try any repurposed candidates with the hope of any improvement of the symptoms (Chen et al., 2019; Elkouzi et al., 2019; Vanle et al.,2018). Another drug first developed as a prophylactic against influenza, Amantadine, is now being used as a medication for treatment of motor complications in PD (Athauda et al., 2018; Thanvi et al., 2004).
Alzheimer’s disease
One of the most devastating diseases among older people is Alzheimer’s disease (AD). AD, or senile dementia, is a disease that has a progressive pattern that effects the memory and other important brain functions. It affects about 3 million people in the United States every year and there is no cure to this day. However, AD symptoms can be reduced with cognition-enhancing medication. There are medications applicable to different stages of the disease, which vary in severity. The current medications to alleviate symptoms of mild to moderate AD are Galantamine, Rivastigmine and Donepezil. All of these are classified as cholinesterase inhibitors and cognitive-enhancing medications, even though the exact mechanism of action is still unclear (Scott and Goa, 2000). Over time as the brain starts to lose its ability to keep producing acetylcholine, the cholinesterase inhibitors start losing effect as well, deeming them useless.
Researchers are trying to stablish a bridge among neurodegenerative/ neurodevelopmental conditions. They all appear to have issues with the glutamatergic system. Glutamate is the major excitatory neurotransmitter in the body, thus an increase in glutamate function can lead to psychiatric as well as somatic distress. Very recently, drugs like Ketamine have been repurposed to treat depression, and as such, it is being used to treat depression in patients suffering from AD or PD, as this is one of the many symptoms associated with these neurodegenerative disorders. To stop the excessive production of glutamate in the brain and avoiding cell death, NMDA receptor (NMDAR) antagonists work to maintain a steady production of glutamate, so that it is not overly produced leading to cell damage and toxicity. NMDAR antagonists, like Ketamine and Memantine, are drugs being considered for repurposing as pain relievers as well as to treat non-motor symptoms of neurodegeneration. Meta-analysis conducted to observe the analgesic effects of NMDA receptor (NMDAR) antagonists using experimental models of acute pain and hyperalgesia have been conducted. Human evoked pain models compared NMDAR antagonists with no intervention. The results showed that pain relief was moderate and required frequent use of the drug to uphold the results (Thompson et al., 2019). NMDAR antagonists are also used as anesthetics for humans and animals, because they can cause a state of dissociative anesthesia. Thus, the use and effects of these medications in AD might be beneficial at least until the disease progress into its final stages (Ballard et al., 2020; Vanle et al.,2018).
For moderate to severe AD, the treatment options available now include Memantine (NMDAR antagonist) or a combination of Donepezil and Memantine. In some cases, these medications could allow for some mental lucidity and attentiveness, with important improvements in the patients, such as being able to use the restroom without needing assistance (Vanle et al., 2018).
There are other five compounds that are being studied for possibly repositioning for AD, and those are tetracycline antibiotics, calcium channel blockers, angiotensin receptor blockers (ARBs), glucagon-like peptide 1 (GLP1) analogues and retinoid therapy (Ballard et al., 2020). From all those, antibiotic therapy, calcium channel blockers and ARBs already yielded negative results. However, GLP1 initially produced a modest result in the frequency of incident dementia, but further studies are promising and provide better evidence that these drugs might prevent incident dementia, especially in people with diabetes (Mullins et al., 2019). Retinoid therapy remains to be adapted into clinical trials. Other new priority compounds are being nominated for further consideration into clinical trials as related to a repurpose for AD. Those are ACE inhibitors (Phenserine), vasodilators (Fasudil), anti-viral drugs (acyclovir and others), and disease-modifying antirheumatic drugs (DMARDs, such as methotrexate, chloroquine phosphate and others) (Ballard et al., 2020). Evidence of benefits is strong for some of them and inconsistent over the studies for some others.
Huntington’s disease
Another neurodegenerative disease is Huntington’s disease (HD), which affects the basal ganglia region of the brain. People diagnosed with HD usually have around 15-20 years after the first symptoms start showing. HD is an autosomal dominant disorder, meaning if one of the parents have the gene, the child would automatically inherit it, and the symptoms may show quite early in life. Genetic testing is available to determine whether the gene is present (McColgan and Tabrizi, 2018). HD symptoms are variable even in range and severity. However, those symptoms grow in strength as time passes and the disease progresses. The symptoms include slow or abnormal eye movements, dystonia (muscle issues with rigidity and contracture), chorea (involuntary jerking of the body), difficulties with balance and posture and problems with speech and swallowing. There is available treatment to alleviate the symptoms, but HD cannot be cured. Since chorea is one of the most recognizable signs when it comes to HD, drugs to reduce monoamine neurotransmission, such as tetrabenazine (TBZ), are dispensed. In fact, this is the only medication that has been approved by the FDA for HD. TBZ is known to hinder vesicular monoamine transporter 2 (VMAT 2) in the central nervous system, resulting in interruption of the packaging of serotonin, dopamine and norepinephrine (Jamwal & Kumar, 2015; Wyant et al., 2017).
Because of the mechanism of action of current approved drugs, researchers are searching for other drugs that also reduce dopaminergic neurotransmitters via D2 receptor blockade or presynaptic depletion. However, these alternative treatments might produce several undesirable side effects such as depressive episodes or suicidal thoughts. As for AD and PD, depression is one of the most prominent effects in patients suffering from HD, but in this case, anti-depressants are not recommended, as they have a detrimental effect (McColgan and Tabrizi, 2018).
Alcohol Use Disorder
Alcohol use disorder is a complex and chronic disease in which, an individual is unable to stop or quit their excessive consumption of alcohol. It has been registered officially in the DSM-5 (The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition). People suffering from alcohol use disorder may experience shakiness, excessive sweating, syncope, compulsive behavior, agitation, delirium and/or lack of restraint. There are different treatments available for those that seek help, with medications, therapies and medical procedures available (Kranzier and Soyka, 2020). In the case of alcohol use disorder, anxiolytic drugs, such as benzodiazepines, are prescribed to treat acute withdrawal symptoms. Some antidepressants, such as selective serotonin reuptake inhibitors (SSRI), may also be prescribed to increase serotonin levels in the brain and impact alcohol consumption.
Other medications like Zonisamide, originally developed as an antiepileptic drug (anticonvulsant), are been considered for alcohol use disorder. This drug appears to maintain sodium channel balance alleviating the after effects of alcohol in these subjects (Arias et al., 2011; Biberdorf et al., 1993; Campbell et al., 2018).
Schizophrenia
Schizophrenia is a psychiatric disorder that affects how a person feel, see and think. The symptoms of this disease include hallucinations, delusions, blunted affect and abolition (no motivation to conduct everyday tasks associated with long-term goals). The cause of this disease is unknown, but genetics, environmental and neurodevelopmental factors are suspected, leading to a change in brain chemical function (Karunakaran et al., 2018). Additionally, research has indicated that inflammation might have a role in schizophrenia (Muller et al., 2015). Regular treatment drugs for schizophrenia are grouped in a class of antipsychotic medication including Thioridazine, Fluphenazine, Prochlorperazine, Clozapine, and Risperidone among many others. Some of those have additional uses unrelated to psychotic illnesses, such as treatment of nausea and vomiting.
Several studies on protein-protein and drug-protein interactions on schizophrenia-associated genes led to drugs that potentially could be repurposed for schizophrenia treatment (Karunakaran et al., 2018). Some of them are drugs originally develop for other psychiatric disorders such as depression (amitriptyline, Nortriptyline). However, many others proposed to study for effectiveness treating schizophrenia are unrelated. Among them, acetazolamide (glaucoma treatment), Alendronate (osteoporosis), alfacalcidol (vitamin D supplement), Amiloride (hypertension), Antazoline (nasal congestion), Bepridil (angina), Cinnarizine and dimenhydrinate (motion sickness), Cromoglicic acid (asthma) Danazol (endometriosis), Miconazole (antifungal), tetracycline (antibiotic used in acne and other conditions). Many of these drugs affect the dopaminergic indicators of schizophrenia, and were proposed to be used in combination with stablished dopamine receptor antagonists, such as clozapine (Karunakaran et al., 2018)
Glutamatergic neuromodulator agents have been studied for schizophrenia. It is well known that antagonists of the NMDA receptor such as ketamine and phencyclidine induce psychotic symptoms (McCutcheon et al., 2020). This has led to a wealth of efforts to repurpose certain drugs affecting the glutamate system for the treatment of schizophrenia. Glutamate modulating treatments including glycine modulatory site (D-serine), agonists of glycine (D-cycloserine, initially used to treat tuberculosis), glycine transport inhibitors (bitopertin) are several examples, some of which yielded disappointing outcomes in clinical trials. Additional research concentrated in the possible repositioning of cyclooxygenase-2 inhibitors (celecoxib, parecoxib and niflumic acid), used to relieve pain and swelling caused by osteoarthritis, post-operative pain, or alleviate inflammation. These cyclooxygenase-2 inhibitors have a potential for greater efficacy by lowering the levels of kynurenic acid, an endogenous antagonist of the glycine modulatory site, thus affecting the glutamate system (Zakrocka et al., 2019). Moreover, another class of medication, benzothiazoles (Riluzole), used to treat amyotrophic lateral sclerosis (ALS), by blocking the release of glutamate, might have a benefit in the treatment of schizophrenia. Likewise, an anti-epileptic drug, lamotrigine, might prove useful by the same mechanism (McCutcheon et al., 2020).
Another drug under study is Varenicline, a drug that blocks α7-subtype of the nicotinic acetylcholine receptor. Varenicline was first approved for smoking cessation, but it has shown positive results improving cognition, verbal learning, executive functions and learning in schizophrenia (Yang et al. 2017).
Depression
Depression is a disorder in which an individual may lose interest in everyday activities with an addition of constant depressive or sad state of mind. This may happen due to biological, physical or psychological distress. It is one of the most common mental disorders among people today. Symptoms may include changes in mood, loss of sleep, weight gain or loss, increased or decreased appetite, self-isolation, decreased energy and over all change in daily behavior. In some extreme cases, depression can lead to suicidal thoughts. The medications prescribed to treat depression are mostly related to the serotonin transmission in the brain, and they are classified as anti-depressant medications.
Some of these drugs have been found suitable for repurposing, benefiting other conditions. For example, tricyclic antidepressants (amitriptyline, nortriptyline) and selective serotonin norepinephrine reuptake inhibitors (duloxetine, venlafaxine), can treat both depression and chronic pain. It appears that the pain is relieved by relaxing the muscles making them effective drugs for pain relief. Duloxetine is a drug that has been also used in chemotherapy-induced peripheral neuropathy for pain management, and it showed promising results improving stress urinary incontinence (Li and Jones, 2012). Moreover, it was also repurposed to treat fibromyalgia and chronic bone or muscle pain (Skånland et al. 2019). Another example is the antidepressant Bupropion, which has been repurposed successfully for smoking cessation (Fava et al., 2005).
Conclusion
The dug repositioning industry seems to be rising as more drugs are finding a new treatment purpose. Sometimes the process is fast, and some others it requires a long period to achieve any result. Nevertheless, drug repurposing is making strides in particularly difficult fields such as oncology and personalized medicine, where it is considered one of the best ways to provide hope. By repurposing, it is easier to get existing drugs approved to treat new conditions, rather than introducing new drugs. The restudy of patented drugs seem to benefit the medical community by shortening the research and development, as well as dispensing and usage.
Neurodegenerative and neurodevelopmental diseases are still incurable. Regrettably, pharmaceutical companies stall research into neuropsychiatric medications due to the lack of perceived profit. However, certain brain medications are seeing additional purposes to treat new conditions, alleviating other disorders not previously considered. Overall, evidence suggest that drug repurposing might be the best way to maximize productivity and profit for drug companies, giving a medication a myriad of options to target a disease, whether it is rare or common.
Acknowledgments
This publication received funding from the underrepresented Women of Color Coalition (UR-WoCC) at the University of Houston.
Conflict of interest and Author contributions
The authors disclose no conflicts of interest or competing interests. Both authors contributed to the design, literature review, and paper preparation.