Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
Voycheva Ch
Department Pharmaceutical Technology and Biopharmacy, Medical University of Sofia, Bulgaria
*Corresponding author: Christina Voycheva, Department Pharmaceutical Technology and Biopharmacy, Medical University of Sofia, Bulgaria.
Received: October 25, 2021
Accepted: November 01, 2021
Published: November 05, 2021
Citation: Voycheva Ch. “Preparation and characterization of Salinomycin loaded amino-functionalized nanodiamond systems with anticancer application”. J Pharmacy and Drug Innovations, 2(5); DOI: http;//doi.org/03.2020/1.1034.
Copyright: © 2021 Christina Voycheva. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Salinomycin loaded amino-functionalized nanodiamonds are prepared by adsorption and pH adjustment. The complex between ND-NH2 and Salinomycin was proved by IR spectroscopy. The resulted and non modified nanoparticles are characterized by dynamic light scattering, and the zeta potential, polydispersity index and size of loaded and nonloaded particles are measured. Finally, ND-NH2 and ND particles were compared by scanning electron microscopy.
Introduction
Nanodiamonds NDs have 4-5 nm size primary particles, stable, inert core, high surface area and tunable surface [1]. They are very attractive from a pharmaceutical point of view as a drug delivery vehicle. They are usually chosen to deliver anticancer drug systems because of their low cost, chemical inertia, and ability to overcome resistance to multiple drugs [2]. One of the problems in using nonmodified nanodiamonds is their spontaneous aggregation and limited dispersion in water. Their modification can be made NDs more hydrophilic and provide better compatibility with aqueous media [3]. Recently, aminated and carboxylated ND improved the dispersibility of drug-loaded delivery systems [4]. Modification of ND surface with amino or carboxyl group helps loading of opposite groups contain drugs. Yu et al. attached Camotothecin alkaloid (Cam) anticancer agent in alkyl-amine modified NDs [5]. They gave high biocompatibility results in vitro and in vivo and higher killing effect than classic Cam–polyethylene glycol (PEG)–micelles and Cam– intralipid emulsions. Moreover, ND loaded with Cam systems showed significantly reduced tumour volumes compared to control measurements in mice.
In another in vitro study, carboxylated NDs caused partial membrane disruption and cell death in a nutritious medium with E. coli growth [6].
Salinomycin kills cancer stem cells in different human cancers by interfering with ABC drug transporters, the Wnt/β-catenin signalling pathway, mitochondrial function and other cancer stem cell (CSC) pathways [7]. Gupta et al. announced that Salinomycin is a 100 times more effective killer of breast cancer stem-like cells than paclitaxel, a commonly used breast cancer chemotherapeutic drug [8]. The mechanism of action by which Salinomycin kills cancer stem cells involves lysosomal iron sequestration, leading to the production of reactive oxygen species, lysosome membrane permeabilization and ferroptosis [9].
Our study aims to attach Salinomycin on the surface of aminated nanodiamonds and to make a preliminary characterization of the resulted system to be used for drug delivery.
Materials
Salinomycin sodium (SAL); amino-functionalized nanodiamond particles in a suspension of 1.5% (ND - NH2) kindly provided to us by Nanotery Ltd.; Ethanol 95% Himmax (Bulgaria); Vanillin - Sigma Aldrich; H2SO4 - Merck (Darmstadt, Germany);
Methods
Loading of amino-functionalized nanodiamond with salinomycin sodium
The medium of the ND – NH2 suspension is pre-adjusted to pH 5.
To 8 ml of a 1.5% suspension of ND-NH2 was added 95% salinomycin sodium 0.01grams, previously dissolved in 2 ml of ethanol. The loading was performed on an electromagnetic stirrer using an ice-cooled water bath at 4 ° C for 2 hours at 500 rpm.
Characterization of salinomycin-loaded amino-functionalized nanodiamond particles.
Method for quantification of loaded salinomycin sodium
The resulting suspension containing nanodiamond-salinomycin complex was centrifuged in an Optima TM LE-80K Ultracentrifuge (Beckman Coulter) at 30,000 rpm for 2 hours. Then, the supernatant is decanted off. The amount of Salinomycin not included in the complexes is determined by spectrophotometry.
The calculation of the amount of free Salinomycin is performed according to a pre-established standard line.
1 ml of the supernatant is mixed with 0.25 vanillin reagent and 2.5 ml of 72% H2SO4. The solution is made up of 25 ml with 95% ethanol. The mixture was incubated for 10 minutes at 60 ° C. Then cool on ice. The resulting complex was measured spectrophotometrically at a wavelength of 530nm with The Thermo Scientific Evolution ™ 300 UV-Vis spectrophotometer.
Infrared spectroscopy (IR)
The IR spectra of samples containing Salinomycin, amino-functionalized nanodiamond particles (ND-NH2) and the complex between amino-functionalized nanodiamond particles and Salinomycin (ND-NH2-SAL), prepared after drying of the diluted dispersions and shot with a Nicolet IS 10 FT-IR in the 4000 and 400 cm-1 range.
Dynamic laser scattering (DLS)
Prepared diluted dispersions of 0.1 / 100 v / v containing nanodiamond particles (ND), amino-functionalized nanodiamond particles (ND-NH2) and the complex between amino-functionalized nanodiamond particles and Salinomycin (ND-NH2-SAL) were subjected to DLS analysis.
The study was performed by dynamic laser light scattering with a Zetasizer Nano ZS device (Malvern Instruments, UK). The device consists of a 632 nm HeNe gas laser and an optical detector equipped with an ITT FW 130 photo magnifier. The particle size distribution and zeta potential were determined by measuring the scattering of the incident beam at an angle of 173 ° C. Three measurements were made for each sample at 25 ° C.
Scanning Electron Microscopy (SEM)
The study was conducted with the device JEOL JSM-5510 (Japan).
Very dilute dispersions of particles are placed on glass dishes and dried in a desiccator under a vacuum. Then, the samples are coated with gold using an instrument (Sputter Coater Jeol Fine Coater 1200, Japan).
Results and discussion
The results obtained by the quantification of loaded Salinomycin, centrifugation, and subsequent UV-Vis spectroscopy, at 530 nm were not included in the complex Salinomycin present in the supernatant.
Loading efficiency (in%) (LE) is calculated by the following formula:
LE = (A-B) / A × 100,
Where A is the total amount of Salinomycin inserted, B is the amount of Salinomycin not included in the complex.
After the measurements and the calculations were made, 100% load was established. From the obtained results, it can be concluded that in addition to the formation of the ND-NH2-SAL complex, there is additional adsorption of Salinomycin on the nanodiamond particles.
Infrared spectroscopy (IR).
|
|
ND-NH2 |
SAL |
ND-NH2-SAL |
|
NH3+ |
1580 см-1, 1599 см-1, 1621 см-1 |
|
1572 см-1, 1583 см-1 и 1610 см-1 |
|
-COOH |
|
1716 см-1 |
1726 см-1 |
|
-COO - |
|
1558 см-1 |
1573 см-1 |
Table 1: shows the results from IR spectra of a) ND-NH2; b) ND-NH2-SAL; c) SAL
The displacement of the characteristic peaks indicates the participation of these groups in a chemical bond. This observation suggests that there is an ionic bond between Salinomycin and ND-NH2. 10
(-NH3 + - OOC)
Dynamic laser scattering (DLS)
The results of the DLS analysis of dilute suspensions to determine the ζ potential, size and particle size distribution are presented in table 2.
|
Sample |
Size, nm |
Polydispersity index |
ζ potential, mV |
|
ND-NH2 |
2039 |
0,043 |
41,4 |
|
ND-NH2-SAL |
1123 |
0,212 |
35,8 |
|
ND |
3831 |
0,265 |
0,87 |
Table 2: Data from DLS and determination of zeta potential of ND-NH2, ND-NH2-SAL, ND
The high values of the average particle size give reason to believe that the measured average size is of aggregates of particles. The polydispersity index shows the distribution of aggregates of particles by size. Salinomycin-loaded amino-functionalized nanodiamond particles show the smallest aggregate size. The size of the formed aggregates is the largest in the non-functionalized nanodiamond particles. The zeta potential value confirms the tendency to form aggregates of non-functionalized nanodiamond particles of 0.87 mV. In the case of amino-functionalized particles, the zeta potential changes to 41.4 mV. The value of the measured zeta potential gives grounds to assume that there is a repulsion between the same charges of the functionalized particles. In the ND-NH2-SAL complex, the value of the zeta potential 35.8 mV shows a greater repulsion between the individual particles and, therefore, smaller size of the formed aggregates.
Scanning Electron Microscopy (SEM)
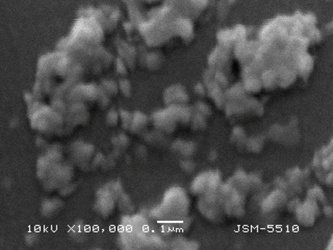

Figure 1. SEM micrography of ND (left) and ND-NH2 (right)
Particle aggregates are observed on the micrographs of ND. In the micrographs of ND- NH2, single particles with dimensions of about 50 – 80 nm can be distinguished, forming smaller aggregates.
Conclusion
Аmino-functionalized nanodiamonds are successfully loaded with salinomycin sodium. The formation of an ionic bond between the anticancer drug and modified nanoparticles is proved by infrared spectroscopy interpretations and allow the modified release of the active substance from the prepared drug delivery system. The zeta potential, polydispersity index and size of the nanodiamonds were compared with nonmodified, and the results showed improved characteristics of ND-NH2. Scanning electron microscopy confirms this observation. In addition, ND-NH2 are less aggregated because of eponymous repulsion charges. The received data demonstrate the potential application of Salinomycin sodium loaded on nanodiamond particles for anticancer application.