Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
Himali J. Prajapati, Manish P. Patel, Praful D. Bharadi and Mansi N. Athalye*
L.M. College of Pharmacy, Opposite Gujarat University, Navrangpura, Ahmedabad, Gujarat-380009, India.
*Corresponding author: Mansi N. Athalye, L.M. College of Pharmacy, Opposite Gujarat University, Navrangpura, Ahmedabad, Gujarat-380009, India.
Received: August 27, 2021
Accepted: September 17, 2021
Published: September 24, 2021
Citation: Himali J. Prajapati, Manish P. Patel, Praful D. Bharadi and Mansi N. Athalye. “Role of Probiotics in Ulcerative Colitis”. J Pharmacy and Drug Innovations, 2(5); DOI: http;//doi.org/03.2020/1.1029.
Copyright: © 2021 Mansi N. Athalye. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Ulcerative colitis (UC) is a common condition resulting in inflammation of the colon. Current treatments for this condition result in side effects in a significant proportion of patients and consequently alternative treatments are being sought. Probiotics are live microorganisms which have been used to treat other inflammatory conditions such as gastroenteritis and pouchitis.
Objective:
In this review, the evidence for the use of probiotics for the treatment of ulcerative colitis has been investigated. The current research suggests that conventional treatment combined with probiotic therapy does not provide any additional benefit over conventional treatment alone in patients with mild to moderate ulcerative colitis. There is limited evidence that probiotics may reduce disease activity. However, there is not enough evidence to recommend the use of probiotics for the treatment of ulcerative colitis.
Conclusion:
Larger, well designed randomised controlled trials are needed to determine whether probiotics are of benefit for the treatment of ulcerative colitis.
Introduction:
Inflammatory bowel disease (IBD) is a chronic recurrent disease, which mainly consists of ulcerative colitis and Crohn’s disease. One of the most common forms of IBD is Ulcerative Colitis (UC), a chronic condition that is characterized by mucosal inflammation and ulcers on the inner lining of the human colon and rectum [3].It is estimated that worldwide 150 out of every 100,000 people suffer from ulcerative colitis [4, 5]. Ulcerative colitis is a common condition resulting in inflammation of the colon [6]. Ulcerative colitis is a chronic, immune-mediated disease of the intestinal tract of which etiology and pathogenesis have not been definitively elaborated [7].Intestinal microflora has long been implicated as an important initiating factor in the pathogenesis of inflammatory bowel disease (IBD)[8].Ulcerative colitis most often begins gradually and can become worse over time. Symptoms can be mild to severe [9]. Most people have periods of remission-times when symptoms disappear - that can last for weeks or years [10]. Ulcerative colitis can occur in people of any age. However, it is more likely to develop in people who are between the ages of 15 and 30, older than 60 and those who have a family member with Inflammatory Bowel Disease [11, 12].
In Ulcerative colitis, the inflammation extends from the rectum in a circumferential manner, typically affecting both sides of the colon in an uninterrupted pattern (Figure 1).
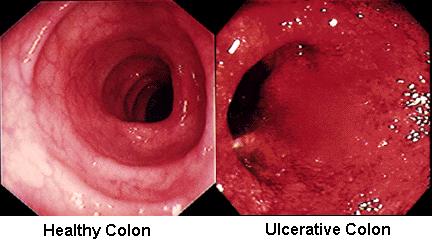
Figure 1: Inflammatory changes in Ulcerative Colitis [13]
Inflammation affects the rectum in over 95% of the cases. Depending on the extent of the inflammation, Ulcerative Colitis can be classified as indicated below and as shown in figure 2 [13, 14]:
inflammation of the rectal mucosa;
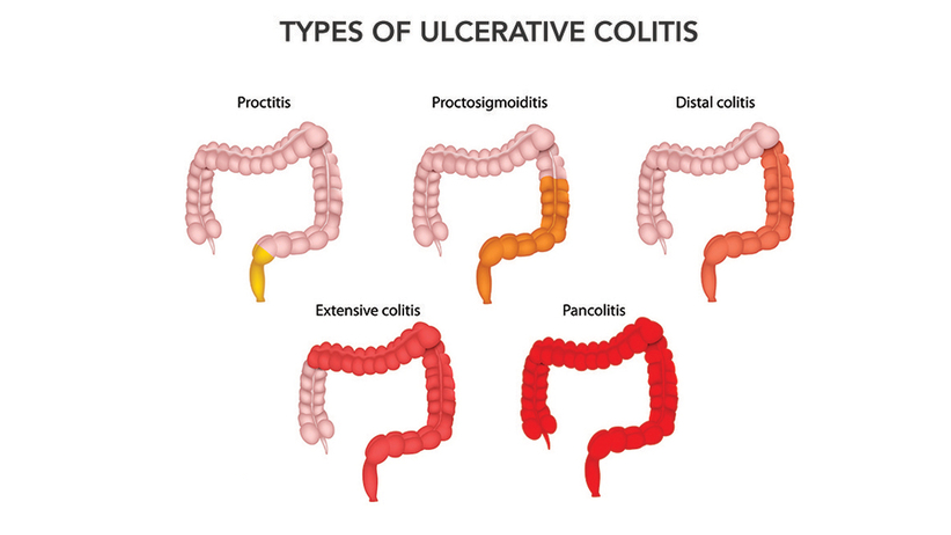
Figure 2: Types of Ulcerative Colitis [14]
UC may also be classified according to symptoms, as either:
The most common signs and symptoms of ulcerative colitis are diarrhoea with blood or pus and abdominal discomfort, rectal bleeding, loss of appetite, fever, weight loss and fatigue. Other signs and symptoms include an urgent need to have a bowel movement, feeling tired, anaemia-a condition in which the body has fewer red blood cells than normal[11].Furthermore, UC may manifest itself outside of the gastrointestinal tract, for instance in the form of episcleritis, uveitis, arthropathy or sclerosing cholangitis[10].
There are various risk factors associated with Ulcerative Colitis such as family history,
abnormal immune response, age, personality type, diet, urban living etc.
The different tests through which Ulcerative Colitis can be diagnosed are blood test, urine test, liver function test, gut test, stool sample, colonoscopy and biopsy.
Primary therapy for Ulcerative Colitis is usually a combination of sulfasalazine and glucocorticoids. Sulfasalazine can be given alone or in combination with other drugs. However, a large number of patients are resistant or intolerant to sulfasalazine. Sulfasalazine, mesalazine, and immune modulators promote remission maintenance, but are not adequately effective. Moreover, an appreciable number of patients cannot tolerate these drugs, and immune modulators can cause serious adverse events. Recently, Probiotic therapy has been acknowledged to be potentially effective and safe in patients with Ulcerative Colitis. The use of probiotics has been proposed for providing benefits to human health for a long time but in recent years there has been increased interest for their use in ulcerative colitis due to the microbiome role in ulcerative colitis pathogenesis. Probiotics are being ingested by patients with ulcerative colitis sometimes through the advice of the physician but mostly self-prescribed as a form of alternative medicine. The reasons for their usage seem to be mostly related to severity of disease, side effects of treatments and health beliefs. Recent reports suggest that patientssufferings from ulcerative colitis are found to be experimenting probiotics about 50% more as compared to earlier [17-18].
Probiotics are defined as a live microbial feed nutritional supplement that beneficially affects the host by improving the balance of the intestinal flora. Studies of animal models of colitis have suggested that the intestinal flora has an important role in the pathogenesis of colitis. Probiotics are live microorganisms which have been used to treat other inflammatory conditions such as gastroenteritis, Ulcerative colitis and pouchitis. Probiotics are viable, non-pathogenic microorganisms that exert health benefits beyond basic nutrition by improving microbial balance. Probiotics have been shown to be safe, as the enterococcal strains used are normal inhabitants of the gastrointestinal tract [8, 24, 25, 26].
Products sold as probiotics include foods (such as yogurt), dietary supplements, and products that aren't used orally, such as skin creams. There are large numbers of microorganisms live on and in our bodies [25].Many of the microorganisms in probiotic products are the same as or similar to microorganisms that naturally live in our bodies[27].Probiotics have also been investigated in relation to atopic eczema and complications of liver cirrhosis[28].Although there is some clinical evidence for the role of probiotics in lowering cholesterol, the results are conflicting[29-31].
The concept behind probiotics was introduced in the early 20th century, when Nobel laureate Elie Metchnikoff, known as the “father of probiotics,” proposed that consuming beneficial microorganisms could improve people’s health. Researchers continued to investigate this idea, and the term “probiotics”—meaning “for life”—eventually came into use. The term probiotic is a relatively new word meaning “for life” and it is currently used to name bacteria associated with beneficial effects for humans and animals. The original observation of the positive role played by some selected bacteria is attributed to Eli Metchnikoff, the Russian born Nobel Prize recipient working at the Pasteur Institute at the beginning of the last century, who suggested that "The dependence of the intestinal microbes on the food makes it possible to adopt measures to modify the flora in our bodies and to replace the harmful microbes by useful microbes" (Metchnikoff, 1907). At this time Henry Tissier, a French paediatrician, observed that children with diarrhoea had in their stools a low number of bacteria characterized by a peculiar, Y shaped morphology. These “bifid” bacteria were, on the contrary, abundant in healthy children (Tissier, 1906). He suggested that these bacteria could be administered to patients with diarrhoea to help restore a healthy gut flora.
The works of Metchnikoff and Tissier were the first to make scientific suggestions about the probiotic use of bacteria, even if the word "probiotic" was not coined until 1960, to name substances produced by microorganisms which promoted the growth of other microorganisms (Lilly and Stillwell, 1965). Fuller (1989), in order to point out the microbial nature of probiotics, redefined the word as "A live microbial feed supplement which beneficially affects the host animal by improving its intestinal balance". A quite similar definition was proposed by Havenaar and Huis in’t Veld (1992) "a viable mono or mixed culture of bacteria which, when applied to animal or man, beneficially affects the host by improving the properties of the indigenous flora". A more recent, but probably not the last definition is "live microorganisms, which when consumed in adequate amounts, confer a health effect on the host" (Guarner and Schaafsma, 1998).
Prebiotics affect intestinal bacteria by increasing the numbers of beneficial anaerobic bacteria and decreasing the population of potentially pathogenic microorganisms. Probiotics affect the intestinal ecosystem by stimulating mucosal immune mechanisms and by stimulating nonimmune mechanisms through antagonism and competition with potential pathogens. These phenomena are thought to mediate most beneficial effects, including reduction of the incidence and severity of diarrhoea, which is one of the most widely, recognized uses for probiotics. Probiotics reduce the risk of colon cancer in animal models, probably due to their role in suppressing the activity of certain bacterial enzymes that may increase the levels of procarcinogens, but this has not been proven in humans.
Probiotics must be able to exert their benefits on the host through growth and/or activity in the human body. However, it is the specificity of the action, not the source of the microorganism that is important. Indeed, it is very difficult to confirm the source of a microorganism. Infants are born with none of these bacteria in the intestine, and the origin of the intestinal microflora has not been fully elucidated. It is the ability to remain viable at the target site and to be effective that should be verified for each potentially probiotic strain. There is a need for refinement of in vitro tests to predict the ability of probiotics to function in humans. The currently available tests are not adequate to predict the functionality of probiotic microorganisms in the intestine.
The various probiotic strains are of Lactobacillus species, Bifidobacterium species and few others are also considered as the probiotic strains, the list of which is mentioned in Table 1.
|
Probiotic strains |
|
Lactobacillus species |
|
|
Bifidobacterium species
|
|
Others
|
Table 1: List of Various Probiotic Strains [37]
The SYNCAN study tested the effect of oligo-fructose plus two probiotic strains in patients at risk of developing colonic cancer. The results of the study suggest that a symbiotic preparation can decrease the expression of biomarkers for colorectal cancer.
It has been confirmed that different probiotic strains, including L. reuteri ATCC 55730, L. rhamnosus GG, L. casei DN-114 001, andSaccharomyces cerevisiae (boulardii) are useful in reducing the severity and duration of acute infectious diarrhoea in children. The oral administration of probiotics shortens the duration of acute diarrheal illness in children by approximately 1 day.
Several meta-analyses of controlled clinical trials have been published that show consistent results in systematic reviews, suggesting that probiotics are safe and effective. The evidence from studies on viral gastroenteritis is more convincing than the evidence on bacterial or parasitic infections. Mechanisms of action are strain-specific: there is evidence for efficacy of some strains of lactobacilli (e.g., Lactobacillus casei GG and Lactobacillus reuteri ATCC 55730) and for Saccharomyces boulardii. The timing of administration is also of importance.
Several lactobacilli and bifidobacterial strains as well as Bacillus clausii, appear to reduce the side effects of antibiotic therapies and improve patient compliance. Several strains were effective in decreasing side effects but did not have effects on the eradication rate. A recent meta-analysis of 14 randomized trials suggests that supplementation of anti-H. pylori antibiotic regimens with certain probiotics may also be effective in increasing eradication rates and may be considered helpful for patients with eradication failure. There is currently insufficient evidence to support the concept that a probiotic alone, without concomitant antibiotic therapy, would be effective. In summary, there is literature suggesting that certain probiotics may be helpful as adjuvant therapy with antibiotics in the eradication of H. pylori infection.
Prebiotics such as lactulose are commonly used for the prevention and treatment of this complication of cirrhosis. Minimal hepatic encephalopathy was reversed in 50% of patients treated with a symbiotic preparation (four probiotic strains and four fermentable fibres, including inulin and resistant starch) for 30 days.
The strongest evidence is for the prevention of atopic dermatitis when certain probiotics are administered to pregnant mothers and newborns up to 6 months of age. However, a recent clinical trial did not confirm these results. With regard to the treatment of allergic disease, a few well-designed studies have provided evidence that specific probiotic strains can be effective in the treatment of a subset of patients with atopic eczema. Little is known about the efficacy of probiotics in preventing food allergy.
Several studies have demonstrated significant therapeutic gains with probiotics in comparison with placebo. A reduction in abdominal bloating and flatulence as a result of probiotic treatments is a consistent finding in published studies; some strains may ameliorate pain and provide global relief (B. infantis 35624) in addition. Lactobacillus reuteri may improve colicky symptoms within one week of treatment, as shown in a recent trial with 90 breastfed babies with infantile colic. In summary, there is literature suggesting that certain probiotics may alleviate symptoms in persons with functional abdominal pain.
There is insufficient evidence to support the use of probiotics and synbiotics in critically ill adult patients in intensive-care units.
The use of probiotics/prebiotics for preventative medicine and decreasing risk of cardiovascular disease is still unproven.
Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricusimprove lactose digestion and reduce symptoms related to lactose intolerance. This was confirmed in a number of controlled studies with individuals consuming yogurt with live cultures.
Clostridium, salmonella, E. coli, etc.
The health benefits of Probiotics and their mechanisms are as indicated in Figure 3.

Figure 3: Health Benefits of Probiotics and Their Proposed Mechanisms [37].
Probiotic preparations consist of 4 strains of Lactobacillus (L. casei, L. plantarum, L. acidophilus, and L. delbrueckii subsp. bulgaricus), 3 strains of Bifidobacteria (B. longim, B. breve and B. infantis), and 1 strain of Streptococcus salivarius subsp. thermophilus. Few of the marketed preparations of probiotics are as mentioned in Table 2.
|
Company |
Probiotic strain |
Marketed product |
|
BioGaia |
L. reuteri |
Drops |
|
BioGaia |
L. reuteri |
Chewable tablets |
|
Nestle |
B. lactis |
Probiotic |
|
Lichu Drug House |
B. adolescentis |
__ |
|
Shanghai Sinyi Drug Pte. Ltd. |
B. longum, B. adolescentis and Enterococcus faecium
|
__ |
|
Mongolian Shuanchi Drug Co. Ltd. |
B. longum and L. delbreuckii subspecies bulgaricus
|
__ |
|
Nestle |
B. lactis |
Baby Cereals |
|
Bio-LiFE |
L. acidophilus LA-5 and B.lactis BB-12 |
A.B.Pre& Pro |
|
Vitis Pharma |
L. rhamnosus GG |
Dicoflor 30 |
|
OptiBac |
L. acidophilus, B. infantis and B. bifidum |
Probiotics |
|
EvoraKidsTM |
B. longum, B. bifidum and B. infantis |
Biomilk with probiotics (Babio) |
|
MXI Corp. TM |
L. helveticus and B. longum |
XoBioticTM Squares |
|
Chocolate Crisp ® |
L. acidophilus, L. casei and B. lactis |
Chocolate probiotic bars |
|
Bunker Hill Cheese Company |
L. acidophilus, L. casei, B. lactis |
Cheese |
Table 2: Marketed Products [45]
Only a few probiotic products either combined as blends or administered as single strain monotherapy have been studied in ulcerative colitis maintenance trials with 3 of the single probiotic trials utilizing E. coli strain Nissle 1917. With a background of up to 70% relapse rate over a 1-year period for those with ulcerative colitis not taking any form of maintenance therapy, many of the trials have been for one year and studied remission rates in comparison with 5-aminosalicylate products. One of these 12-month probiotic versus 5-aminosalicylate trials was initiated with active ulcerative colitis patients and followed those achieving remission for a 12-month period. In this study the relapse rates were high in both the group maintained with E. coli Nissle 1917 and those maintained on 1.5 g of daily mesalazine (67% and 72%, respectively). The other 12-month trials were initiated in participants with quiescent disease. In these studies, maintenance of remission rates varied between 45% and 75% and studies in those receiving 5-aminosalicylates as a control group had a similar maintenance of remission rate as the probiotic intervention group. Interestingly in the trial comparing monotherapy L. rhamnosus strain GG, monotherapy mesalamine (2.4 g per day) andcombination probiotic and mesalamine, no synergistic benefit was derived from combination therapy, but all three groups had equivalent rates for maintenance of remission. The studies comparing probiotic with 5-aminosalicylates have used different total daily amounts (1.5–2.4 g per day). Nevertheless, currently there is not currently clinical evidence of a direct dose-dependent maintenance benefit above 1.6 g daily dosing of 5-aminosalicylate.
In spite of inherent difficulties establishing good measures of probiotic efficacy, studies on lactose intolerance, diarrhoea and colon cancer show that a daily dose of about 10" - 10'"(1-10 billion) bacteria is needed for any measurable effect. Unfortunately, the concentration of probiotics in food products varies tremendously and there are currently no national standards of identity for levels of bacteria required in yogurt or other fermented products. Concentration of bacteria contained in food products is generally left up to the manufacturer, who may or may not accept recommendations from industry groups. The “Live active culture” seal established by the National Yogurt Association requires 108 viable lactic acid bacteria per gram at the time of manufacture for refrigerated yogurt and 107 per gram for frozen yogurts. However, these counts may not accurately indicate probiotic content as they do not differentiate probiotic bacteria from starter culture bacteria such as S. thermopliilus. Culture manufacturers recommend including approximately 106 probiotic bacteria per gram for yogurt and acidophilus milk at the end of shelf-life. As research in this field progresses and consumers demand tighter regulation, requirements for probiotic concentration and viable counts at the time of consumption will undoubtedly become more standardized for these products [43].
Due to their long history of use in food fermentation, the FDA has designated many probiotics to be generally recognized as safe (GRAS). Even for those without GRAS status, the industry has used probiotic bacteria in food fermentations with the assumption that their history of use implies their safety. The occasional reports of bacteremias and endocarditis associated with Lactobacillus have generally been in severely immunocompromised individuals. Epidemiological data on the safety of dairy products and a thorough review of the safety data on probiotics suggests no evidence of probiotics being involved with human infections. However, there always remains the possibility that probiotic consumption can cause infection and that individuals will respond in different ways to a specific probiotic strain. The food industry will need to carefully assess the safety and efficacy of all new species and strains of probiotics before incorporating them into food products. As a practitioner it may be prudent to advise clients to incorporate well-known species into their diet gradually, building up to the recommended daily levels needed over a period of two to three weeks, to minimize any potential deleterious effects.
From a scientific perspective, the suitable description of a probiotic product asreflected on the label should include [33]:
Since probiotics contain live microorganisms, concurrent administration of antibiotics could kill a large number of the organisms, reducing the efficacy of the Lactobacillus and Bifidobacterium species. Patients should be instructed to separate administration of antibiotics from these bacteria-derivedprobiotics by at least two hours. Similarly, S. boulardii might interact with antifungals, reducing the efficacy of this probiotic. Probiotics should also be used cautiously in patients taking immunosuppressants, such as cyclosporine, tacrolimus, azathioprine, and chemotherapeutic agents, since probiotics could cause an infection or pathogenic colonization in immunocompromised patients.
In spite of the problems with dosage and viability of probiotic strains, lack of industry standardization and potential safety issues, there is obviously considerable potential for the benefits of probiotics over a wide range of clinical conditions. On- going basic research will continue to identify and characterize existing strains of probiotics, identify strain-specific outcomes, determine optimal doses needed for certain results and assess their stability through processing and digestion. Gene technology will certainly play a role in developing new strains, with gene sequencing allowing for an increased understanding of mechanisms and functionality of probiotics. In addition to such basic research, industry-centered research will focus on prolonging the shelf-life and likelihood of survival through the intestinal tract, optimizing adhesion capacity and developing proper production, handling and packaging procedures to ensure that the desired benefits are delivered to the consumer.
Randomised Controlled Trials (RCTs) is to identify comparative studies of probiotics in ulcerative colitis.
Twenty patients (12 male and 8 females, mean age40 years; range 30±65), intolerant or allergic to oral 5-aminosalicyclic acid (5-ASA), with ulcerative colitis in remission, have been studied. Patients are eligible for the treatment for mild to moderate ulcerative colitis as indicated in Table 3 which ranged from a minimum of 3 to a maximum of 11 and who had duration of exacerbated symptoms lasting less than 4 weeks. Patients infected with enteric pathogen, diagnosed with CD or pouchitis, were excluded. Patients who received a high dose of oral prednisone (>10 mg/day) within the last 4 weeks or antibiotics within the last 2 weeks prior to entry were excluded. Patients who had a change in dose of oral 5-aminosalicyclicacid (5-ASA) containing products within the last4 weeks or a change in dose of rectal 5-ASA or steroids within 7 days prior to study entry were also excluded. Patients with known hepatic, renal, endocrine, respiratory, neurological, or cardiovascular diseases or patients who required imminent surgery or those who had a severe disease as defined by an SCCAI of 12 or greater were excluded. In addition, patients who were pregnant or lactating were also excluded.
|
Simple clinical colitis activity index |
|
|
Symptoms |
Score |
|
Bowel frequency (day) |
|
|
1-3 |
0 |
|
4-6 |
1 |
|
7-9 |
2 |
|
>9 |
3 |
|
Bowel frequency (night) |
|
|
1-3 |
1 |
|
4-6 |
2 |
|
Urgency of defecation |
|
|
Hurry |
1 |
|
Immediately |
2 |
|
Incontinence |
3 |
|
Blood in stool |
|
|
Trace |
1 |
|
Occasionally frank |
2 |
|
Usually frank |
3 |
|
General well being |
|
|
Very well |
0 |
|
Slightly below par |
1 |
|
Poor |
2 |
|
Very poor |
3 |
|
Terrible |
4 |
|
Extracolonic features |
|
|
Uveitis, Pyoderma, Gangrenosum erythema nodosum, Arthropathy |
1 per manifestation |
Table 3: Ulcerative Colitis Activity Index [8, 56]
Concurrent medications were recorded, and patients were allowed to continue to maintain a stable dose of oral5-aminosalicyclicacid (5-ASA) and a low dose of corticosteroids, provided dosage was stable for at least 4 weeks prior to study entry. Patients receiving stable doses of immunosuppressive medications such as azathioprine or 6-mercaptopurine for at least 3months prior to entry were eligible. Non-steroidal anti-inflammatory drugs (NSAIDs) and antidiarrheal agents (loperamide, diphenoxylate, and opiates) were not permitted throughout the 8-week trial.
Patients were treated twice daily for 8 weeks with a dose of probiotic based on their age. The dose of probiotics for age is determined by scaling doses shown to be efficacious and safe for the treatment of ulcerative colitis in the adult trial as recommended by the manufacturer. The concentration of bacteria used in this trial exceeded previous pediatric trials by10–20 billion per dose.
Subjects were seen at baseline, weeks 2, 4, and 8 and were asked to record in a diary their daily symptoms, adverse events, and medications taken. Subjects were asked to record stool frequency, urgency of defecation, signs of blood in stool, extra-colonic manifestations, and general well-being. At baseline and final (week 8) visit colonoscopy/sigmoidoscopy and histologic assessment of disease activity were determined .Blood, urine, and stool were collected pre- and post-treatment for hematology (a complete blood count with differential and erythrocyte sedimentation rate (ESR); biochemistry [electrolytes, creatinine, alkaline phosphates, albumin, and C-reactive protein (CRP)], and urinalysis [pH, protein, glucose, ketone, blood, and microscopic sediment examination].A stool sample was obtained to exclude infection(including Clostridium difficile toxin assay, bacterial pathogen culture, and parasite examination). Blood serum was collected pre- and post-treatment for the cytokine profile (tumor necrosis factor-alpha [TNF-ἀ], BD Biosciences, San Jose, CA); interferon-gamma (IFN-ᵧ) (R & D Systems, Minneapolis, MN) using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. Pre- and post-rectal biopsies were also collected for microbial profiling based on bacterial signatures encoded in 16S ribosomal RNA.
The colonoscopy/sigmoidoscopy assessment was based on the Mayo endoscopic scoring system for ulcerative colitis ranging from mild, moderate, to severe (1–3, respectively).
8. Conclusion:
Ulcerative colitis (UC) is a chronic disease and easy to relapse, its etiology and pathogenesis have not been definitively elaborated. The pathogenesis of UC is closely related to intestinal microbiota, although the exact bacteria that contribute to UC have not been determined. The use of probiotics in UC is currently being investigated. Probiotics may help normalize the imbalance of intestinal microbiota, improve the micro ecological environment, enhance intestinal mucosal barrier function, and reduce gastrointestinal infections. Use of probiotics in UC is provocative and suggests potential for benefit in selected patients but concerns remain about the evidences of treatment from trials.
List of Abbreviations:
UC: Ulcerative Colitis
IBD: Inflammatory Bowel Disease
L.: Lactobacillus
B.: Bifidobacterium
SYNCAN: Synbiotics and Cancer Prevention in Humans
ATCC: American Type Culture Collection
FDA: Food and Drug Administration
GRAS: Generally Recognized As safe
RCTs: Randomized Controlled Trials
ASA: Amino salicylic Acid
SCCAI: Simple Clinical Colitis Activity Index
NSAIDs: Non-Steroidal Anti-Inflammatory drug
ESR: Erythrocyte Sedimentation Rate
CRP: C-Reactive Protien
ELISA: Enzyme Linked Immunosorbent Assay
Declaration of Interest:
The authors report no conflict of interest.
Acknowledgement:
I would like to thank all my teachers for their encouragement and motivation. I would also like to thank L. M. College of Pharmacy for providing me facilities to compile the data for this review article.