Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
B. Vijaya kumar*, N. Supraja
Hi-tech Pharma R & D Biotech Division, Nellore-524401, A.P., India Nanotechnology laboratory, Acharya N G Ranga Agricultural University, Regional Agricultural Research Station, Tirupathi-517502, A.P., India.
*Corresponding author: B. Vijaya kumar, Hi-tech Pharma R & D Biotech Division, Nellore-524401, A.P., India Nanotechnology laboratory, Acharya N G Ranga Agricultural University, Regional Agricultural Research Station, Tirupathi-517502, A.P., India.
Received: August 26, 2021
Accepted: September 17, 2021
Published: September 24, 2021
Citation: B. Vijaya kumar, N. Supraja. “A mini Review on Role of Probiotics used in Aquaculture”. J Pharmacy and Drug Innovations, 2(5); DOI: http;//doi.org/03.2020/1.1028.
Copyright: © 2021 B. Vijaya kumar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The need to improve food production to meet the necessities of ever-increasing population is an immediate challenge of today. Aquaculture is one area that has increased over the recent years. But aquaculture is not itself free of limitations, with disease outbreak as one of the major constraints. The need to increase disease resistance and enhance feed efficiency has brought in the use of Probiotics as non-antibiotic agent in aquaculture productions. The documented evidences do suggest that Probiotics can improve water quality, secretion of growth promoters, disease resistance, and enhancement of immune response. The field of Probiotics as well as the selection steps to acquire probiotic strain for the management of disease in aquaculture has been discussed. This report provides a summary of probiotic application and significance in aquaculture.
Introduction:
Aquaculture as an industry has seen tremendous growth in recent years seeking out economic centre-stage [1]. It is considered to be the fastest growing food-producing sector in the world today with a potential to meet the growing demand for aquatic food [2]. The fish-farming industry has a major caveat due to increased intensification and commercialization - Disease [5]. The usage of anti-microbial agents has been a successful method to increase productivity, but not for long. The significant problems of using these agents are accumulation of residues of these compounds in farmed fish thus can enter humans, micro-biota gaining resistance to these compounds, and also killing of the beneficial micro-biota in the guts of fish as well [6]. Taking into consideration the above risks these compounds pose; it has warranted that antibiotics must be used with utmost care [7]. In view of the risk associated with the use of antibiotics, the development of non-antibiotic agents is one of the key factors for health management in aquaculture. According to Browdy [8], one of the most significant technologies that evolved as an alternative for the management of the problem is usage of Probiotics. The term probiotic means beneficial organisms; it was derived from two Greek words ‘pro’ and ‘bios’ [9]. Probiotics are live microbes that can be used to improve the host intestinal microbial balance and growth performance. Development of Probiotics for aquaculture management will reduce the dependence on antimicrobial agents that were just prophylactic and pose health hazards to the consuming humans [10]. This report summarizes the selection processes, application, and significance of Probiotics in aquaculture.
Selection of Probiotics:
The primary objective of using Probiotics is to maintain or reestablish a favorable relationship between friendly and pathogenic microorganism that constitute the flora of intestinal or skin mucus of fish. The successful Probiotics are expected to have a set of specific properties in order to be certified for their beneficial effects. The proper selection and commercialization of Probiotics follows the process as depicted in the flow chart below in (Figure 1)
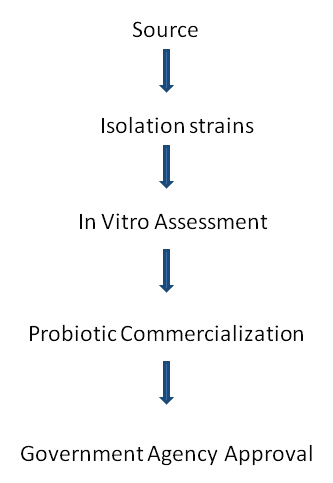
Figure 1: Flow chart for selection of Probiotics.
Beneficial strains of microorganisms from the digestive tract of healthy aquatic animals must be selected. They must further be selectively isolated, identified and sub-cultured. A new culture with only the colonies of interest for conducting in vitro evaluations such as inhibition of pathogens pathogenicity to target species; resistance conditions of host; among others are performed. In case of the absence of restrictions on the use of the target species, experiments with in vivo supplementation, and small and large scale, are carried out to check if there are real benefits to the host. The culture that exhibits significantly positive traits upon each stage of selection is finalized for Probiotics and approved for commercialization. Characteristics of good Probiotics Fuller [11] listed the following as features of good probiotic bacteria:
Any organism that features the above characteristics will show considerable advantage over antibiotics. However, they do not induce any resistance to compromise therapy in necessary cases. They would be non-toxic and will not accumulate in the fish, hence promoting healthy growth of fish and will not harm consuming humans through a food chain. They may stimulate immunity whereas the immune status remains unaffected for antibiotics.
Constraints to Probiotics in aquaculture:
Probiotics significance in aquaculture:
There are some possible benefits linked to the administering of Probiotics which may have already been suggested as:
Improvement in water qualities:
In fish culture, contamination of culture systems/ponds with nitrogenous compounds such as ammonia, nitrite and nitrate has been a serious concern. High accumulation of these compounds in the culture water bodies over-time might prove unhealthy to the fish, which are generally not so harmful in smaller quantities. Ma et al. [12] reported the ability of Lactobacillus spp. JK-8 and JK-11 in removing nitrogen and pathogens from contaminated shrimp farms. In several other studies, water quality has been improved by the addition of Probiotics especially Bacillus spp. [13,14]. The reason is that gram-positive Bacillus spp. according to Stanier et al. [15] are generally more efficient in converting organic matter back to CO2 than gram – negative bacteria, which would convert a greater percentage of organic carbon to bacterial biomass or slime.
As growth promoters:
It has been demonstrated experimentally that probiotics indeed may enhance the growth of fish. The ability of the probiotic organisms is that they outgrow the growth of pathogenic microbes, which in turn would create a form of host-friendly biotic environment. So not many negative effects are found on fish in culture thus increasing the yield. Yassir et al. [16] in attempt to use probiotic bacteria as growth promoter on Tilapia (Oreochromis niloticus) identified that the highest growth performance was recorded with Micrococcus luteus a probiotic and the best feed conversion ratio was observed with the same organism. So, M. luteus may be considered as growth promoters in fish aquaculture. Also, Lactic acid bacteria had shown a positive effect on the growth of juvenile carp - although the effect could not be reproduced with Sea bass [17].
For disease prevention:
Probiotics or products there-of were found to have associated health benefits in aquaculture, terrestrial animals, and in human disease control. Probiotics function as microbial adjuncts and act against pathogens colonizing and proliferating in the intestinal tract of hosts, and also control undesired pathogenic growth on the superficial surfaces of the culture species (hosts) [13]. The probiotics play a major role in optimizing the immune response system of culture species by either increasing their resistance to manifestations of disease itself, or by producing inhibitory substances against colonizing of pathogenic species inside and outside host.
Source of nutrients and enzymatic contribution to digestion:
It has been reported by multiple groups that some micro-organisms significantly increase the digestive system of aquatic animals. In Fish, Bacteroides and Clostridium sp. contribute to the host’s nutrition, by synthesizing and releasing into its digestive tract - fatty acids and vitamins [18]. Some microorganisms such as Agrobacterium sp., Pseudomonas sp., Brevi-bacterium sp., Microbacterium sp., and Staphylococcus sp. may contribute to nutritional processes in Salvelinus alpinus L [19].
Enhancement of the immune response:
Among the numerous beneficial effects of probiotics, modulation of immune system is one of the most commonly purported benefits of probiotics.
Probiotics in aquaculture management:
The mode of administration of probiotics to the aqua-culture species can be done through direct-feeding, a mere dissolution into the culture ponds, or through injection [21].
Application as direct feed:
Probiotics are supplied as feed along with a binder (egg or cod liver oil), and most commercial preparations contain either Lactobacillus sp or Saccharomyces cerevisiae [22]. FAO and WHO guidelines stipulate that probiotic organisms that are used in food must be capable of surviving passages through the gut i.e., they must have the ability to resist gastric juices and exposure to bile [23]. Furthermore, they must also be able to safely colonize the digestive tract and proliferate as well. They must also have a potential shelf life for effective usability [23].
Direct application to pond water:
Multiple strains of bacteria can be used for direct application in water, like Bacillus acidophilus, B. subtilis B. lecheniformis, Nitrobacter sp, Aerobacter and Sacharomyces cerevisiae. Application of probiotics to water bodies of aqua-culture also show significant changes in the physic-chemical properties of water in context of culture animal health, since they change the biome of the water altogether [24, 25].
Application through injection:
Application of probiotics by injection is also a possibility. Austin et al., [26] suggested the application either through bathing, or injection, after freezing the probiont like in case of a vaccine. Yassir et al. [16] has demonstrated the experimental administration of probiotic Micrococcus luteus to Oreochromis niloticus by injection through intra peritoneal route, which had only 25% mortality as against 90% with Pseudomonas using the same route. According to Yassir et al. [16, 27] has observed on usage of probiotics, that they stimulate a Rainbow trout immunity by stimulating phagocytic activity that involves complement mediated bacterial killing and immunoglobulin production [17].
Conclusion:
Increased use of antibiotics has led to the high proportion of antibiotic-resistant bacteria, which provide threat to fish and man through consumption of the infected fish. Inefficiencies in antibiotic treatment of fish illnesses lead to significant economic losses. But the use of probiotics in aquaculture has shown to have beneficial impact on fish health and thereby economic performance of fish farming. At the same time, the use of probiotics has also important environmental benefits. By reducing the risk of diseases, the necessity of medication and thereby the risk of residues left in the environment is reduced. Therefore, the use of probiotics in fish feed should also be seen as an important step in aquaculture sustainability.
Recently research on probiotics has focused on identification of bioactive ingredients and compounds extracted from probiotic bacteria. This should help provide a clearer picture of the mode of action of probiotics in maintaining gastrointestinal health. However, much more work is needed to elucidate the potential benefits.
Recommendations:
Fish farmers and other stakeholders in aquaculture management should make use of probiotics because of its colonization ability - as preventive measures against over dependency on antibiotic therapy, which is both costly and unsafe. Fish farmers are also encouraged to incorporate probiotics in their feed formulations because of its importance in digestibility improvement. Close network of aquaculture experts, and fish nutrition centers must be established. The rise in bacterial antibiotic resistance and antibiotic residues in cultured aquatic animals due to extensive use of chemotherapeutic agents has become a global concern. Vaccination and immune stimulant treatment are ideal methods for preventing infectious diseases, but their use remains very limited and rather uncommon in aquaculture, especially in Southeast Asia.
Conflict of Interest:
Authors not having any Conflict of Interest
Acknowledgement:
I would like to thank to Hi-Tech Pharma, C.E.O. Ramana Reddy, Nellore