Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
Ceela Swetha, Chandragiri Bhavani Shankar, Dabbi Sravanthi, Dandaboina Pooja, Vasudha Bakshi, Narender Boggula, Jainendra Kumar Battineni*
Department of Pharmaceutical Chemistry, School of Pharmacy, Anurag University, Venkatapur, Ghatkesar, Hyderabad, Telangana, India.
*Corresponding author: Jainendra Kumar Battineni, Department of Pharmaceutical Chemistry, School of Pharmacy, Anurag University, Venkatapur, Ghatkesar, Hyderabad, Telangana, India.
Received: July 21, 2021
Accepted: August 16, 2021
Published: August 18, 2021
Citation: C Swetha, Ch Bhavani Shankar, D Sravanthi, D Pooja, Vasudha B, Narender B, Jainendra Kumar B. “Assessment of Phytochemical Evaluation and Antibacterial Activity of Mirabilis Jalapa - An in Vitro Design”. J Pharmacy and Drug Innovations, 2(5); DOI: http;//doi.org/03.2020/1.1024.
Copyright: © 2021 Jainendra Kumar Battineni. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Plants are making use of completely as raw drugs for many formulations in traditional systems of medicine. To check the genuineness of the raw drugs and to detect adulteration of these materials, an authentic pharmacognostic study is required for each raw drug. Usually, the drugs are collected by traditional practitioners who have inherited Ayurveda or other herbal practices. The aim of project work is to assess the phytochemical screening, in-vitro antibacterial activity of Mirabilis jalapa leaves using ethanol as a solvent. The antibacterial activity was assessed by cup plate method and disc diffusion method. Streptomycin was used as standard drug. Preliminary phytochemical investigation of the leaf extract exhibited the existence of glycosides, flavonoids, tannins and saponins. Results reveal that ethanolic extract of Mirabilis jalapa leaves were significantly effective against gram positive, gram negative bacteria. The zone of inhibition is measured to be the positive result for the given samples by these two methods. Phytochemical results indicate that Mirabilis jalapa have different type of alkaloids, terpenes, carbohydrates, proteins and amino acids in various concentrations in leaves. Our aim is to find plants which have antibacterial activity without many side effects. A detailed study needs to be carried out to isolate bioactive compounds that show antibacterial activity.
Introduction
Man has been using herbs and plant products for combating diseases since times immemorial. The Indian subcontinent is enriched by a variety of flora-both aromatic and medicinal plants. This is used in a wide diversity of climatic conditions in India ranging from deserts to swamp lands. Numerous types of herbs have been well recognized and catalogued by botanists from the high ranges of the Himalayan tract up to the sea shore Kanyakumari. This extensive flora has been greatly utilized as a source of many drugs in the Indian traditional system of medicine [1,2].
In India, the earliest mention of the use of medicinal plants was found in the Rigveda which was written between 4500-1600 BCE. A detailed account of the world's first symposium on medicinal plants is given the first chapter of Brihat Samhita and since 1600 BCE the amount of literature on the subject is boundless. The traditional system of medicine is so engrained in our culture that, even now, 75% of the Indian population depend on this indigenous system for relief [3]. With such a huge section of an ever-increasing population relying on herbal remedies, it is imperative that the plant products which have been in use for such a long time be scientifically supported for their efficacy [4].
Throughout the world, about 35,000-70,000 species of plants have been used at one time or another medicinal, nutraceuticals and cosmeceuticals purposes. In India, about 1,000 plant species, in Nepal about 700 species, about 700 species in the Peninsula and Malaysia and its neighbouring islands and in Chinese medicine about 9,905 plant materials are used but only a relatively very small number of them are used in any significant volume [5]. According to the International Trade Centre (ITC) report, there is generally upward trend except for 1990, when it dipped slightly before rising again to US $ 1.08 billion in 1991. The world trade in medicinal plants and raw material from plant parts averaged US$ 1.28 billion during 1995-1999 [6].
Mirabilis jalapa, the marvel of Peru or four o'clock flower, is the most commonly grown ornamental species of Mirabilis plant, and is available in a range of colours. Mirabilis in Latin means wonderful and Jalapa (or Xalapa) is the state capital of Veracruz in México. Mirabilis jalapa was cultivated by the Aztecs for medicinal and ornamental purposes. The flowers usually open from late afternoon or at dusk (namely between 4 and 8 o'clock), giving rise to one of its common names. Flowers then produce a strong, sweet-smelling fragrance throughout the night, and then close for good in the morning (showed in Figure 1 and 2). New flowers open the following day. It arrived in Europe in 1525. Today, it is common in many tropical regions and is also valued in Europe as a (not hardy) ornamental plant [7,8].

Figure 1: Mirabilis jalapa plant

Figure 2: Mirabilis jalapa leaves
The aim of the present project work is to assess the phytochemical screening, in-vitro antibacterial activity of Mirabilis jalapa leaves using ethanol as a solvent.
Materials and Methods
Collection of plant material
The plant material was collected from surrounding areas in Ghatkesar, Hyderabad, Telangana, India. The fresh leaves were collected and washed with tap water and latter with deionized water and dried under shade. The plant material was regularly checked for fungal growth or rotting. After the plant material was dried it was powdered with the help of an electric blender and sieved through size 80 sieve to obtain a uniform fine particle size. This plant material was stored in airtight containers at 4 0C for future usage [9].
Preparation of plant extract
200g of the sieved powder was accurately weighed and extracted with solvent like ethanol in a Soxhlet extractor (illustrated in the following Figure 3) for 72 h. The extract thus obtained was concentrated under reduced pressure to yield crude plant extract. The extract was stored at 4 0C in amber coloured glass stoppered vials for further use [10,11].

Figure 3: Extraction of leaves by soxhlation
Qualitative Phytochemical Evaluation [12,13]
The different chemical tests were performed for establishing profile of the extract for its chemical composition; the following chemical tests for various phytoconstituents in the ethanol extract was carried out as described below.
(A) Test for alkaloids:
i) Dragendorff’s Test: In a test tube containing 1ml of extract, few drops of Dragendorff’s reagent was added and the colour developed was noticed. Appearance of orange colour indicates the presence of alkaloids.
ii) Wagner's Test: To the extract, 2 ml of Wagner's reagent was added; the formation of a reddish-brown precipitate indicates the presence of alkaloids.
iii) Mayer's Test: To the extract, 2 ml of Mayer's reagent was added, a dull white precipitate revealed the presence of alkaloids.
iv) Hager's Test: To the extract, 2 ml of Hager's reagent was added; the formation of yellow precipitate confirmed the presence of alkaloids.
(B) Test for terpenoids:
i) Salkowski test: To 1 ml of extract, tin (one bit) and thionyl chloride were added. Appearance of pink colour indicates the presence of terpenoids.
ii) Hirshonn reaction: When the substance was heated with trichloroacetic acid, red to purple colour was observed.
(C) Test for steroids:
i) Liebermann Burchard Test: To 1ml of extract, 1ml of glacial acetic acid and 1ml of acetic anhydride and two drops of concentrated sulphuric acid were added. The solution become red, then blue and finally bluish green indicates the presence of steroids.
(D) Test for coumarins:
i) To 1 ml of extract, 1 ml of 10% sodium hydroxide was added. The presence of coumarins is indicated by the formation of yellow colour.
(E) Test for tannins:
i) To few mg of extract, ferric chloride was added, formation of a dark blue or greenish black colour showed the presence of tannins.
ii) The extract was mixed with basic lead acetate solution; formation of white precipitate indicated the presence of tannins.
(F) Test for saponins:
i) To 1 ml of the extract, 5 ml of water was added and the tube was shaken vigorously. Copious lather formation indicates the presence of saponins.
(G) Test for flavones:
i) Shinoda Test: To the extract, a few magnesium turnings and 2 drops of concentrated hydrochloric acid were added, formation of red colour showed the presence of flavones.
ii) To the extract, 10% sodium hydroxide or ammonia was added; dark yellow colour shows the presence of flavones.
(H) Test for quinones:
i) To 1 ml of the extract 1 ml of concentrated sulphuric acid was added. Formation of red colour shows the presence of quinones.
(I) Test for flavanones:
i) To the extract, 10% sodium hydroxide was added and the colour changes from yellow to orange, which indicates the presence of flavanones.
ii) To the extract, conc. sulphuric acid was added, and the colour changes from orange to crimson red, which indicates the presence of flavanones.
(J) Test for anthocyanins:
i) To the extract, 10% sodium hydroxide was added, and the blue colour shows the presence of anthocyanins.
ii) To the extract, conc. sulphuric acid was added, and the yellowish orange colour confirms the presence of anthocyanins.
(K) Test for anthraquinones:
i) Borntrager's test: The extract was macerated with ether and after filtration; aqueous ammonia or caustic soda was added. Pink red or violet colour in the aqueous layer after shaking indicates the presence of anthraquinones.
(L) Test for phenols:
i) Ferric chloride test: To the extract, few drops of 10 % aqueous ferric chloride were added. Appearance of blue or green colour indicates the presence of phenols.
(M) Test for proteins:
i) Biuret Test: To the extract, 1 ml of 40% sodium hydroxide solution and two drops of one percent copper sulphate solution were added. Formation of violet colour indicates the presence of proteins.
ii) Xanthoprotein Test: To the extract, 1 ml of concentrated nitric acid was added. A white precipitate was formed; it is then boiled and cooled. Then, 20% sodium hydroxide or ammonia was added. Orange colour indicates the presence of aromatic amino acids.
iii) Tannic Acid Test: To the extract, 10% tannic acid was added. Formation of white precipitate indicates the presence of proteins.
(N) Test for carbohydrates:
i) Molisch's Test: To the extract, 1 ml of alpha-naphthol solution, and concentrated sulphuric acid through the sides of test tube were added. Purple or reddish violet colour at the junction of the two liquids revealed the presence of carbohydrates.
ii) Fehling's Test: To the extract, equal quantities of fehling's solution A and B were added and on heating, formation of a brick red precipitate indicates the presence of carbohydrates.
iii) Benedict's Test: To 5 ml of Benedict's reagent, extract was added and boiled for two minutes and cooled. Formation of red precipitate showed the presence of carbohydrates.
(O) Test for amino acids:
i) Ninhydrin test: Two drops of ninhydrin solution were added to the extract, a characteristic purple colour indicates the presence of amino acids.
(P) Test for fixed oils and fats:
i) Spot Test: A small quantity of extract was pressed between two filter papers. Oil stains on the paper indicates the presence of fixed oils and fats.
(Q) Test for volatile oils:
i) To the section of drug, add alcoholic solution of Sudan III. Formation of red colour obtained by globules indicates the presence of volatile oils.
ii) To the thin section of drug, add a drop of tincture alkaline. Formation of red colour indicates the presence of volatile oils.
iii) To the test sample, add 1% Osmic acid. Formation of black colour indicates the presence of volatile oils.
In-vitro antibacterial activity screening [14-16]
Cup plate method:
Prepare stock solution of the antibiotic as 1000µg/1ml (1mg/1ml). Prepare dilutions of the antibiotic of known concentration of the standard and the test antibiotic to be examined. Sterilize the Muller-Hinton agar medium in an autoclave at 121 0C at 15lbs pressure for 15 min. Add 1ml suspension of standard test organism to Muller Hinton medium and mix thoroughly while maintaining temperature at 50 0C. Pour the above mixture into petri dish to form a layer of about 3mm thickness. Allow the medium to solidify. Now cut the reservoirs/cup with sharp tool such as cork borer. Remove the cylindrical plugs with scalpel or sharp forceps. Mark the cups as per dilutions and add in each cup the respective dilutions of the anti-biotic. Keep the plate carefully in the refrigerator for diffusion of anti-biotic for 20 min. Wipe the condensed water carefully from the lid of the petri dish, with the sterile cotton plugs. Incubate the petri dish at 37 0C for 18-24 h.
Disc diffusion method:
Select a pure culture plate of one of the organisms to be tested. Aseptically emulsify a colony from the plate in the sterile saline solution. Mix it thoroughly to ensure that no solid material from the colony is visible in the saline solution. Repeat until the turbidity of the saline solution visually match that of the standard turbidity. Take a sterile swab and dip it into the broth culture of organism. Gently squeeze the swab against the inside of the tube in order to remove excess fluid in the swab. Take a sterile Mueller-Hinton agar (MHA) plate or a nutrient agar (NA) plate. Use the swab with the test organism to streak an MHA plate or a NA plate for a lawn of growth. After the streaking is complete, allow the plate to dry for 5 min. Antibiotic discs can be placed on the surface of the agar using sterilized forceps. Gently press the discs onto the surface of the agar using flame sterilized forceps or inoculation loop. Carefully invert the inoculated plates and incubate for 24 h at 37 0C. After incubation, use a metric ruler to measure the diameter of the zone of inhibition for each antibiotic used. Compare the measurement obtained from the individual antibiotics with the standard table to determine the sensitivity zone. Compare the measurement obtained from the individual antibiotics to the standard table to determine whether the tested bacterial species is sensitive or resistant to the tested anti-biotic.
Results and Discussion
Phytochemical investigation
The ethanolic extract of M. jalapa leaves were subjected to qualitative chemical tests to determine the chemical constituents present in the extract like alkaloids, terpenoids, glycosides, tannins and flavonoids etc. were illustrated in Table 1.
|
Constituents |
Ethanol extract |
|
Terpenoids |
+ |
|
Saponins |
+ |
|
Steroids |
+ |
|
Phenols |
- |
|
Flavonoids |
+ |
|
Coumarins |
- |
|
Carbohydrates |
+ |
|
Alkaloids |
+ |
|
Quinones |
- |
|
Tannins |
+ |
|
Proteins |
+ |
|
Oils & fats |
- |
|
Anthraquinones |
+ |
|
Anthocyanins |
- |
|
Amino acids |
- |
|
Volatile oils |
- |
+ indicates present, - indicates absent
Table 1: Preliminary phytochemical screening of M. jalapa leaves
In-vitro antibacterial activity
Antibacterial assay of the ethanol extract of dried leaves of M. jalapa exhibited dose dependent antibacterial activity against the tested micro-organisms at three different concentrations. The potential sensitivity of the extract was obtained against all the tested micro-organisms and the zone of inhibition was recorded and presented in the following tables and figures given below (Table 2 and 3, Figure 4 and 5).
The antibacterial assay was done by cup plate method and disc diffusion method with the observations of Zone of Inhibition (ZoI) at different concentrations (given in Table 4 and 5, Figure 6-9).
Cup plate method:
|
S. No. |
Name of microorganism |
Concentration (µg/1ml) |
||
|
10 |
20 |
30 |
||
|
|
|
Zone of Inhibition (cm) |
||
|
1 |
Escherichia coli |
2.5 |
2 |
2.7 |
|
2 |
Klebsiella pneumonia |
0.5 |
1.5 |
1.5 |
|
3 |
Staphylococcus aureus |
1.5 |
1.3 |
1.7 |
|
4 |
Bacillus subtilis |
1.6 |
1.5 |
1.8 |
Table 2: Zone of inhibition shown by the standard drug (streptomycin)

Figure 4: Zone of inhibition shown by the standard drug (streptomycin)
|
S. No. |
Name of microorganism |
Concentration (µg/1ml) |
||
|
10 |
20 |
30 |
||
|
|
|
Zone of Inhibition (cm) |
||
|
1 |
Escherichia coli |
1.4 |
2.5 |
2.6 |
|
2 |
Klebsiella pneumonia |
1.4 |
1.6 |
1 |
|
3 |
Staphylococcus aureus |
1.2 |
1.4 |
1.6 |
|
4 |
Bacillus subtilis |
2 |
2.2 |
2.5 |
Table 3: Zone of inhibition shown by the ethanolic extract of M. jalapa leaves 
Figure 5: Zone of inhibition shown by the ethanolic extract of M. jalapa leaves A) Escherichia coli B) Klebsiella pneumonia C) Staphylococcus aureus D) Bacillus subtilis
Disc diffusion method:
|
S. No. |
Name of microorganism |
10µg/1ml |
20µg/1ml |
30µg/1ml |
|
1 |
Escherichia coli |
2.5cm |
2 cm |
2.7 cm |
|
2 |
Klebsiella pneumonia |
0.5 cm |
1.5 cm |
1.5 cm |
|
3 |
Staphylococcus aureus |
1.5 cm |
1.3 cm |
1.7 cm |
|
4 |
Bacillus subtilis |
1.6 cm |
1.5 cm |
1.8 cm |
Table 4: Zone of inhibition shown by the standard drug (streptomycin)
|
S. No. |
Name of microorganism |
20µg/1ml |
40µg/1ml |
60µg/1ml |
|
1 |
Escherichia coli |
1.5 cm |
1.2 cm |
1.38 cm |
|
2 |
Klebsiella pneumonia |
1.38 cm |
1.3 cm |
1.5 cm |
|
3 |
Staphylococcus aureus |
1.5 cm |
1.6 cm |
1.8 cm |
|
4 |
Bacillus subtilis |
1.7 cm |
1.5 cm |
1.5 cm |
Table 5: Zone of inhibition shown by the ethanolic extract of M. jalapa leaves 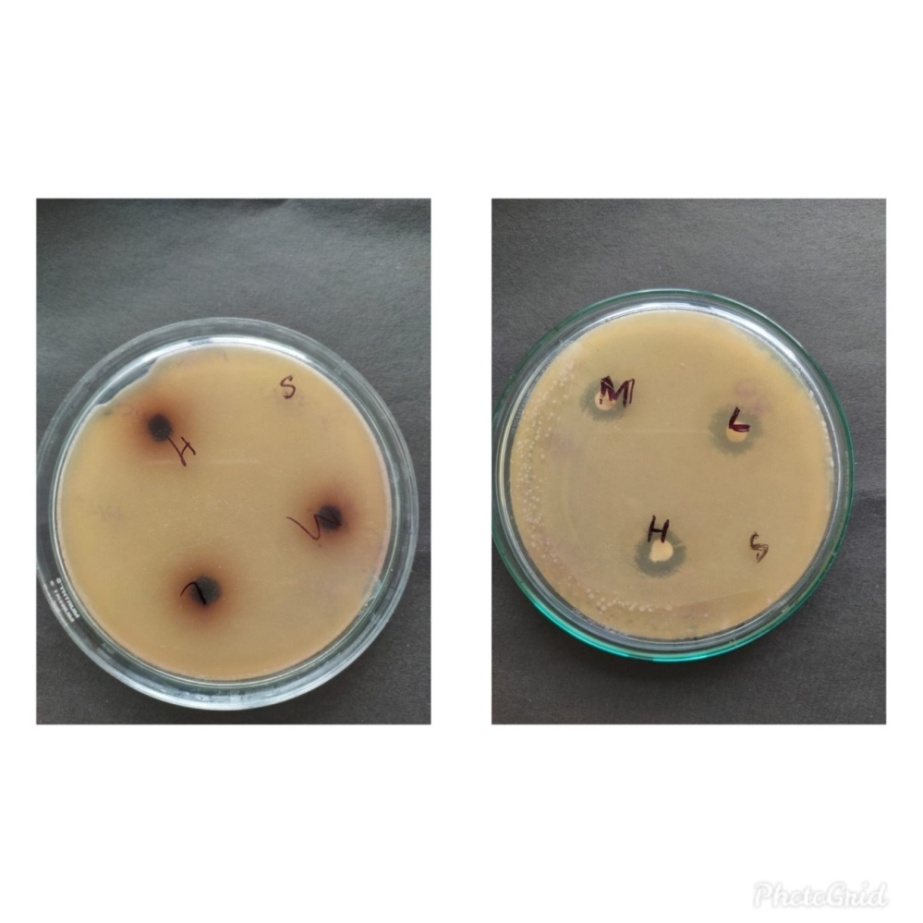
Figure 6: Zone of inhibition of Staphylococcus aureus
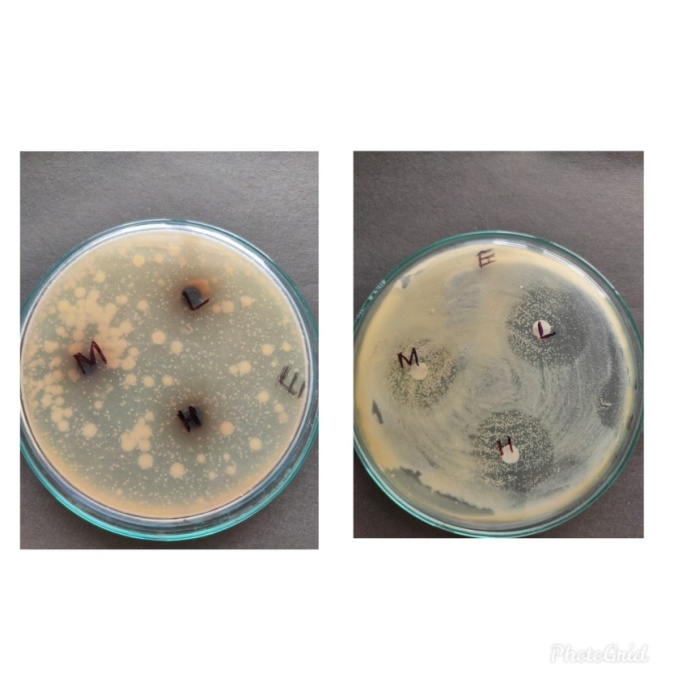
Figure 7: Zone of inhibition of Escherichia coli
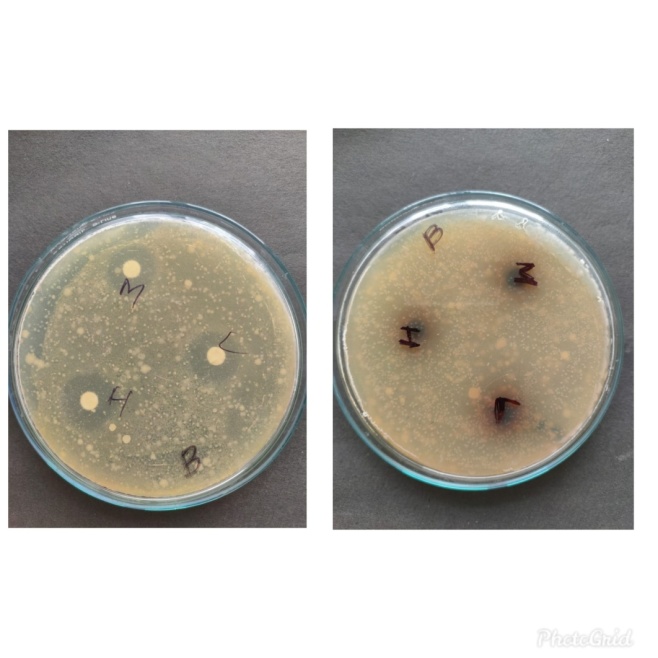
Figure 8: Zone of inhibition of Bacillus subtilis
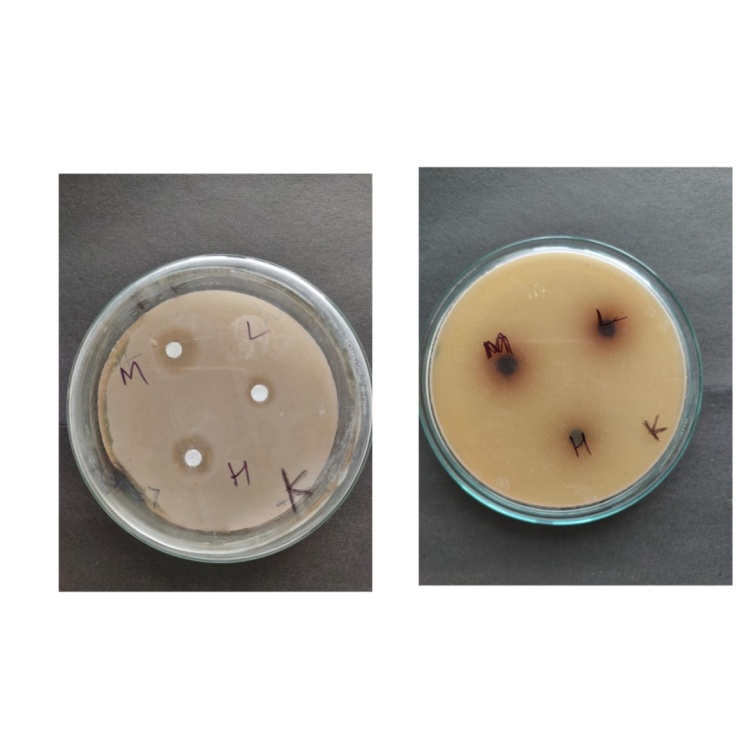
Figure 9: Zone of inhibition of Klebsiella pneumonia
Plants are important source of pharmacophore which will function as new chemotherapeutic agents. The first step to develop a chemotherapeutic agent from plants would be the assay of in-vitro antibacterial activity. The extracts thus found active will help to identify the active compounds responsible for the activities from the plant. In recent years multi drug resistance is seen in pathogenic bacteria which has revived interest in the search of new antibacterial agents from natural sources. In fact, gram negative bacteria P. aeruginosa are frequently reported to have developed multi drug resistance to many of the anti-biotics. But the extract shows a good activity against bacterial strains. The antibacterial agents from natural sources also eliminate the side effects of synthetic or semi synthetic antibacterial agents. The antibacterial activity of the plant extract was variable with various organisms.
Results reveal that ethanolic extract of Mirabilis jalapa leaves were significantly effective against gram positive, gram negative bacteria. The antibacterial activity of the given samples is tested using cup-plate (or) cylinder plate method and disc diffusion method. The zone of inhibition is considered to be the positive result for the given samples. The antibacterial activity is tested by using the microbial strains mentioned in the above tables.
The samples exhibited positive test results are as following for the ethanolic leaf extract of Mirabilis jalapa. Preliminary phytoconstituents screening of the extract showed the presence of glycoside, flavonoids, tannins and saponins. Thus, further work can be carried out on the isolation. In addition, these results confirmed the evidence in previous studies, which reported that ethanol is a better solvent for more consistent extraction of antibacterial substances from medicinal plants compared to other solvents.
Conclusion
Mirabilis jalapa has been medicinally used as a therapeutic agent for a variety of diseases. Moreover, numerous research works have proven its uses beyond the medicinal ones in experimental animals. Alkaloids and flavonoids which were isolated from this plant may be responsible for its pharmacological activities. Therefore, the cultivation, collection, and further pharmacological exploration of Mirabilis jalapa are essential.
The antibacterial activity of the samples is assessed using the different concentration of the sample i.e., low, intermediate and high. The present investigation reveals that the zone of inhibition is found in the sample mentioned above in the tabular column i.e., Mirabilis jalapa ethanolic leaf extract. The standard drug streptomycin is found to be very effective anti-microbial agent. Here it is found that the standard drug show antibacterial activity on both Gram +ve and –ve bacteria and it is found that the zone of inhibition increased as the concentration of the sample increased.
The findings of this study support the view, that the ethanolic extracts of plants are promising sources of potential antibacterial and may be efficient as preventive agents in some diseases and can be considered as a natural herbal source in pharmaceutical industry. Further detailed studies on isolation of phytoconstituents of the plant extracts are essential to characterize them as biological antibacterial agents. This knowledge about the medicinal plant’s usage can also be extended to other fields like field of pharmacology. In view of the nature of the plant, more research work can be done on humans so that a drug with multifarious effects will be available in the future market. Furthermore, a detailed and systematic approach can be done in exploiting and identifying the phytopharmacology to explore in knowing the maximum potentiality of the plant which will be useful to mankind.
Acknowledgement
We express our indebtedness and sense of gratitude the management of School of Pharmacy, Anurag University, Venkatapur, Ghatkesar, Hyderabad, Telangana, India for providing the necessary facilities, constant encouragement, praiseworthy inspiration and support.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Competing interest statement
The authors declare there is no conflict of interest.
Additional information
No additional information is available for this paper.