Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
Marc Lapointe 1*, Jessica Ho 2, Paul J. Nietert 3
1Medical University of South Carolina, South Carolina College of Pharmacy, Department of Clinical Pharmacy and Outcomes Sciences; College of Medicine, Department of Neurosciences.
2Wishard Health Services, Department of Pharmacy Services, Indianapolis, Indiana.
3Medical University of South Carolina, Division of Biostatistics and Epidemiology.
*Corresponding author: Marc Lapointe, South Carolina College of Pharmacy Medical University of South Carolina, 280 Calhoun Street, Suite QF-413, Charleston, SC 29425
Received: July 20, 2021
Accepted: July 25, 2021
Published: July 30, 2021
Citation: Lapointe M, Jessica Ho, Paul J. Nietert. “Green Blend of Silver Nanoparticles by Utilizing Andrographis Echioides Leaf Fluid Remove and Their Antibacterial Action”. J Pharmacy and Drug Innovations, 2(5); DOI: http;//doi.org/03.2020/1.1023.
Copyright: © 2021 Marc Lapointe. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction: Intravenous (IV) antihypertensive therapy (AHT) is commonly required to achieve blood pressure (BP) control before administering IV tissue plasminogen activator (tPA). However, the associated time delays and the intensity of IV AHT have yet to be described.
Methods: An observational, nonrandomized, retrospective evaluation of the management of severe uncontrolled hypertension prior to IV tPA was conducted to characterize the use of IV AHT, and to measure associated time delays. A cohort of 33 patients who required IV AHT for thrombolysis was compared to a control group of 94 patients with no AHT. Patient characteristics, hospital factors, standard of care determinants and metrics were evaluated to measure time delays, and the intensity of IV AHT used.
Results : The adjusted median door-to-needle time for IV tPA was longer among the AHT group (79 minutes vs. 69 minutes, p=0.027). The median number of doses or titrations of antihypertensive agents given before and during IV thrombolysis was 3, and it was significantly greater for the AHT subgroup with recorded systolic BP greater than 200 mm Hg (6 vs. 3, p< 0.05). Overall, polypharmacy was required for the AHT group in 48%.
Conclusion: IV AHT is a determinant that can significantly delay the administration of IV tPA. The intensity of IV AHT required and the use of polypharmacy are clinically significant. Clinicians should initially anticipate more aggressive IV AHT when systolic BP is greater than 200 mm Hg so that door-to-needle time is not further delayed by BP control.
Introduction
Severe uncontrolled hypertension during acute ischemic stroke (AIS) is a relatively common occurrence in nearly 20% of patients [1-3]. Patients who present early to the emergency department (ED) may be eligible for intravenous (IV) thrombolysis with tissue plasminogen activator (tPA) if it can be administered within 4.5 hours of stroke onset [4, 5]. However, the presence of severe uncontrolled hypertension, defined as a systolic blood pressure (SBP) > 185 mm Hg or a diastolic blood pressure (DBP) > 110 mm Hg, is an absolute contraindication to IV thrombolysis [5]. Consequently, IV antihypertensive therapy (AHT) prior to administering IV tPA is often required to achieve and maintain adequate BP control. Guidelines from the American Heart Association (AHA) offer limited and non-specific recommendations to clinicians because of a lack of evidence-based research for this clinical dilemma. Optimal BP management strategies have yet to be defined for severe uncontrolled hypertension in neurological patients, particularly for AIS. Standardized IV AHT and optimal targeted BP remain controversial [6].
When IV AHT is indicated for AIS, it should be administered cautiously in order to prevent abrupt declines in BP that could compromise perfusion within the ischemic penumbra, lead to infarct progression and neurological worsening [5]. Severe uncontrolled hypertension is also recognized as a potential factor for increased risk of hemorrhagic conversion. Thus, appropriate BP management during AIS in patients eligible for IV tPA is critical before, during, and after the administration of thrombolysis [7].
At our institution, we noted a few cases of potentially significant delays and the use of polypharmacy IV AHT during the management of severe uncontrolled hypertension prior to and during IV tPA administration. These cases prompted us to further study these clinical observations. Two large population-based studies identified extreme hypertension as a possible non-time related exclusion for IV tPA in general, but limited descriptions were provided [2, 3]. Another large national registry of AIS patients treated with IV tPA within three hours of symptom onset evaluated patient characteristics, hospital factors, and outcomes associated with successful door-to-needle times (DTT) within 60 minutes of ED arrival. Unfortunately, the need for IV AHT and the presence of severe hypertension were not specifically evaluated [8]. Another publication evaluated two BP control management practices and found significant delays in DTT when severe hypertension required aggressive BP lowering treatments with nicardipine versus monotherapy with labetalol [9]. However, this report did not further describe the intensity of IV AHT used. To our knowledge, no other report has specifically evaluated the extent of these time delays, and described BP control management in AIS patients requiring IV AHT before, during, or immediately after IV thrombolysis. We deemed that a specifically designed study was necessary to better understand the practical aspect of this clinical dilemma.
The purpose of this study was to characterize the use of IV AHT during acute BP management, and to measure the potential time delays caused by treating severe uncontrolled hypertension in a cohort of AIS patients who received IV tPA. Our study also aimed to further describe the intensity of IV AHT used, and evaluate potential safety concerns related to the acute management of BP in the setting of AIS and IV thrombolysis.
Methods
An observational, retrospective evaluation of BP management during AIS was conducted at a university medical hospital with a stroke program accredited by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO). Consecutive patients were identified through a uniform data query of the institution’s stroke center database. All patients who received IV tPA for a diagnosis of AIS between May 1, 2006 and April 30, 2012 were eligible for analysis. Patients were excluded if their medical records were incomplete or if they experienced any concurrent medical complications during the management of the AIS. Exclusionary complications included cardiac resuscitation, hypoxia, respiratory arrest, seizure, complicated intubation, or any other medical conditions that could have altered or delayed the current standard of care for AIS. Likewise, subjects were excluded if they received an MRI or MRA study prior to thrombolysis, had a diagnostic CT performed at an outside hospital, or were already hospitalized at the time of stroke onset. Those exclusions were necessary to minimize factors that could inherently delay or shorten the standard of care for AIS.
Study subjects were categorized into one of the two study groups for comparison. Patients who did not require a reduction in BP for IV tPA eligibility were categorized into the “Control” group. Subjects who required any IV antihypertensive agents prior to or during the IV infusion of tPA were categorized into the AHT group. The AHT group was also further divided into two subgroups for analysis based on when IV AHT was first initiated in relation to the IV tPA infusion. Specifically, the AHT Before subgroup received the initial dose of an IV antihypertensive medication prior to the tPA bolus, and the AHT During subgroup first received IV AHT during tPA infusion. These AHT subgroups were necessary to further differentiate the effect of IV AHT on door-to-needle time (AHT Before vs. Control) and the overall intensity of IV AHT at given steps to achieve or maintain IV tPA eligibility (AHT Before and AHT During vs. Control).
A data collection tool was developed and pilot tested to gather all medically relevant information for the study. Data abstraction was independently completed by two investigators and cross-verified by the second investigator for discrepancies and accuracy. Relevant data regarding stroke standard of care included demographics (i.e. age, gender, race) and past medical history (i.e. ischemic stroke, hypertension, renal disease defined as glomerular filtration rate < 60 mL/min, coronary artery disease, and congestive heart failure). Additional data collected included relevant laboratory values and concomitant medications associated with potential contraindications for IV thrombolysis. Other relevant standard of care determinants related to stroke severity, BP upon ED arrival, and timing variables and outcomes that could explain any differences between the two study groups were also collected and evaluated. Those determinants included time of admission in relation to time of stroke onset and regular working hours (defined as Monday through Friday from 7AM-5PM), time to CT scan, time to draw laboratory studies, time and type of imaging studies performed, need for intubation prior to thrombolysis, hemorrhagic complications, early neurological worsening, in hospital death, and discharge disposition.
We evaluated the intensity of IV AHT by recording the number of different IV antihypertensive medications used, and the number of bolus doses or titrations of continuous infusion necessary before, during, and up to three hours after the end of the infusion of tPA. We also examined medical records for any episodes of clinically significant hypotension and the use of fluid boluses or vasopressors, which could have lead to an abrupt discontinuation of IV AHT.
During the study period, our institution had a CT scan in the ED where all stroke patients received their imaging studies. Preprinted and electronic order sets also were available to reinforce AHA recommendations on BP management during AIS in IV tPA candidates (i.e. administering IV AHT for SBP > 185 mm Hg or DBP > 110 mm Hg). The study was conducted with the approval of the local institutional review board at the Medical University of South Carolina.
Statistical analyses for comparability were performed between the AHT and Control groups. Although not reported in the tables, these same analyses were also conducted between the Control group and the AHT Before subgroup since they were used to evaluate time variables and delays in IV thrombolysis. For the purpose of this study report, any significant differences or similarities between the Control group and the AHT Before subgroup are summarized in the text. Of note, all AHT subjects were included when describing the intensity of IV AHT used for the purpose of IV thrombolysis
Statistical analyses were performed with a commercially available software (SAS System version 9.3; SAS, Inc, Cary, North Carolina). Continuous variables were characterizes using the median and interquartile range and compared between groups using Wilcoxon rank sum tests. Categorical variables were characterized using percentages analyzed using the Chi-square test or Fisher’s exact test, as appropriate. Continuous time-to-event variables for outcomes were analyzed using Log-Rank tests (for univariate comparisons) and Cox proportional hazards models (for multivariable associations).
Results
A total of 127 subjects met the eligibility criteria and received IV tPA for AIS during the study period. A total of nine patients within the Control group were excluded for analysis because they either required an MRI prior to IV thrombolysis (n=2), were already hospitalized at the time of their stroke (n=6), or experienced severe agitation that limited the conduct of radiological imaging (n=1). None of these exclusion criteria applied to the AHT group. However, one patient in the AHT group was excluded because he had a diagnostic CT done at an outside hospital.
The baseline characteristics of all subjects included in the study are presented in Table 1.
|
Variables |
Control Group (n = 94) |
AHT Group (n = 33) |
P |
|
Age, median (IQR) |
67 (54, 78) |
66 (54, 80) |
0.81 |
|
Female sex |
48 (51%) |
14 (42%) |
0.39 |
|
Race |
|||
|
Caucasian |
49 (52%) |
21 (64%) |
0.25 |
|
Non-White (African American & Others) |
45 (48%) |
12 (36%) |
0.25 |
|
Laboratory values |
|||
|
NIHSS score, median (IQR) |
10 (7, 18) |
8 (6, 14) |
0.28 |
|
Serum glucose, median (IQR), mg/dL |
115 (99, 143) |
110 (93, 148) |
0.57 |
|
Platelets, median (IQR), K/mL |
223 (181, 290) |
237 (195, 282) |
0.39 |
|
INR, median (IQR) |
1.06 (0.99, 1.15) |
1.00 (0.96, 1.06) |
0.04 |
|
aPTT, median (IQR), seconds |
27.4 (25.6, 29.8) |
27.2 (25.4, 29.2) |
0.74 |
|
Serum creatinine, median (IQR), mg/dL |
1 (0.8, 1.2) |
1.1 (0.9, 1.3) |
0.16 |
|
Past medical history |
|||
|
Stroke/TIA |
22 (23%) |
7 (21%) |
0.80 |
|
Diabetes |
22 (23%) |
8 (24%) |
0.92 |
|
Hypertension |
67 (71%) |
30 (91%) |
0.02 |
|
Coronary artery disease |
17 (18%) |
6 (18%) |
0.99 |
|
Atrial fibrillation |
27 (29%) |
9 (27%) |
0.87 |
|
Congestive heart failure |
20 (21%) |
1 (3%) |
0.01 |
|
Renal disease |
12 (13%) |
6 (18%) |
0.44 |
|
Smoking |
28 (30%) |
10 (30%) |
0.96 |
|
Preexisting disability |
10 (11%) |
4 (12%) |
0.76 |
|
Medications on admission |
51 (54%) |
21 (64%) |
0.35 |
|
Antiplatelets |
37 (39%) |
13 (39%) |
0.99 |
|
Warfarin |
8 (9%) |
5 (15%) |
0.22 |
|
Other anticoagulants |
1 (1%) |
0 (0%) |
0.99 |
|
Antihypertensive agents |
51 (54%) |
21 (64%) |
0.35 |
|
Blood pressure at time of ED arrival |
|||
|
Systolic, median (IQR), mm Hg |
144 (129, 155) |
189 (166, 199) |
<0.0001 |
|
Systolic BP ≥ 200 mm Hg at any time |
0 (0%) |
12 (36%) |
|
|
Diastolic, median (IQR), mm Hg |
78 (69, 90) |
94 (85, 107) |
<0.0001 |
|
|
|
|
|
|
aPTT activated partial thromboplastin time, BP = blood pressure, CT computed tomography, CTA = CT angiography, CTP CT perfusion, INR international normalized ratio, IQR interquartile range, TIA transient ischemic attack |
|||
Table 1: Patient characteristics
A total of 94 subjects (Control group) did not require a reduction in BP for IV tPA eligibility while 33 subjects required IV AHT prior to or during IV thrombolysis. Overall, 23 subjects required BP reduction for IV tPA eligibility (AHT Before subgroup) and an additional 10 subjects received the first dose of IV AHT during IV tPA infusion to maintain BP control (AHT During subgroup).
Patient characteristics including demographic data, clinical laboratory data, stroke severity, and concomitant therapies were similar between the Control and AHT groups. Along with higher SBP and DBP upon ED arrival, as expected, a history of hypertension was also documented more frequently in the AHT group (91% vs. 71%, p=0.02). The only other difference found between the groups was congestive heart failure, which was more prevalent in the Control group (21% vs. 3%, p=0.01) compared to the AHT group and INR value, which was significantly lower (p=0.04) in the AHT group but not clinically significant. Similar findings were observed when we compared the AHT Before subgroup to the Control group.
Hospital factors are described in Table 2.
|
Variables |
Control Group (n = 94) |
AHT Group (n = 33) |
P |
|
ED arrival after regular working hoursa |
50 (53%) |
15 (45%) |
0.44 |
|
Transfer from outside hospital |
7 (7%) |
5 (15%) |
0.30 |
|
Intubation in ED before IV tPA |
1 (1%) |
3 (9%) |
0.054 |
|
Imaging studies |
|||
|
CT alone |
12 (13%) |
3 (9%) |
0.57 |
|
CT/CTA/CTP |
82 (87%) |
30 (91%) |
0.57 |
|
Timing variables |
|||
|
Stroke-to-Arrival, median (IQR), minutes |
68 (46, 105) |
74 (58, 104) |
0.45 |
|
Door-to-CT, median (IQR), minutes |
22 (15, 34) |
26 (17, 31) |
0.50 |
|
CT-to-Needle, median (IQR), minutes |
43 (32, 60) |
45 (35, 59) |
0.88 |
|
Laboratory-to-Needle, median (IQR), minutes |
60 (46, 78) |
60 (53, 75) |
0.57 |
|
|
|
|
|
|
CT computed tomography, CTA computed tomography angiography, CTP computed tomography perfusion, ED emergency department, IQR interquartile range a Regular hours: Monday through Friday from 07:00 to 17:00 |
|||
Table 2: Hospital factors and standard of care determinants
No differences were observed between the Control and the AHT Before groups when analyzing all the clinically relevant timing variables. Similarly, neither group was more likely to be transferred from an outside hospital or to arrive to the ED beyond regular working hours. The only moderate difference found was related to the slightly greater incidence of intubation prior to IV tPA in the AHT Before group significant (9% vs. 1%, p=0.054). Clinical outcomes pertaining to discharge disposition, medical complications and interventions during hospitalization were also comparable between groups (Table 3). Of interest, the rate of hemorrhagic conversion within 36 hours of thrombolysis was less than 5% in all groups. No cases of clinically significant hypotension requiring intervention were identified in the AHT group.
|
Variables |
Control Group (n = 94) |
AHT Group (n = 33) |
P |
|
Discharge disposition |
|||
|
Home |
50 (53%) |
14 (42%) |
0.29 |
|
Rehabilitation |
26 (28%) |
11 (33%) |
0.54 |
|
Nursing home |
13 (14%) |
5 (15%) |
0.99 |
|
Death during hospitalization |
8 (9%) |
3 (9%) |
0.99 |
|
Clinical outcomes |
|||
|
Neurological decline |
6 (6%) |
4 (12%) |
0.28 |
|
Hemorrhagic conversion |
3 (3%) |
1 (3%) |
0.99 |
|
Intra-arterial intervention |
14 (15%) |
5 (15%) |
0.99 |
|
Clinically significant hypotension |
0 (0%) |
0 (0%) |
0.99 |
|
|
|
|
|
Table 3: Clinical outcomes
Standard of care metrics
The standard of care metrics in both groups are described in Table 4.
|
Variables |
Control Group (n = 94) |
AHT Before (n = 23) |
P |
|
Standard of care metrics |
|||
|
Unadjusted door-to-needle time, median (IQR), minutes |
69 (59, 79) |
79 (69, 89) |
0.19 |
|
Adjusted door-to-needle timea, median (IQR), minutes |
69 (59, 79) |
79 (69, 89) |
0.027 |
|
IV tPA bolus given within 60 minutes of ED arrival |
34 (37%) |
7 (30%) |
0.69 |
|
IV AHT intensity before and during IV tPA |
|||
|
≥ 3 doses or titrations |
N/A |
14/23 (61%) |
|
|
≥ 3 doses or titrations when SBP > 200 mm Hg |
N/A |
11/12 (92%) |
|
|
|
|
|
|
|
AHT antihypertensive therapy, ED emergency department, IQR interquartile range, IV intravenous, N/A not applicable, tPA tissue plasminogen activator |
|||
|
aAdjusted for stroke-to-arrival time |
|||
Table 4: Results
The median DTT was longer for the AHT Before group than that of the Control group (79 minutes vs. 69 minutes, p=0.19), although this unadjusted difference was not statistically significant. However, there was a correlation between the stroke-to-arrival time and DTT (p<0.001) suggesting that subjects who presented late to the ED were more likely to experience a shorter DTT time than those who presented early on after stroke onset. Consequently, after adjusting for stroke-to-arrival time (using a Cox survival model), the median DTT was significantly shorter among the Control group when compared to the AHT Before group (p=0.027). The proportion of subjects receiving IV thrombolysis within 60 minutes of ED arrival was somewhat greater for the Control group (37% vs. 30%), but it did not reach statistical significance (p=0.54).
Intensity of IV AHT
From the entire cohort of subjects who received IV tPA, 26% required BP control between ED arrival and the end of tPA infusion. Specifically, 18% of subjects required IV AHT prior to thrombolysis. On average, these subjects required a median of 2 bolus doses or titrations (range 1-8) before the initial tPA bolus and a median of 3 doses or titrations (range 1-10) before or during the IV tPA infusion (Fig. 1).
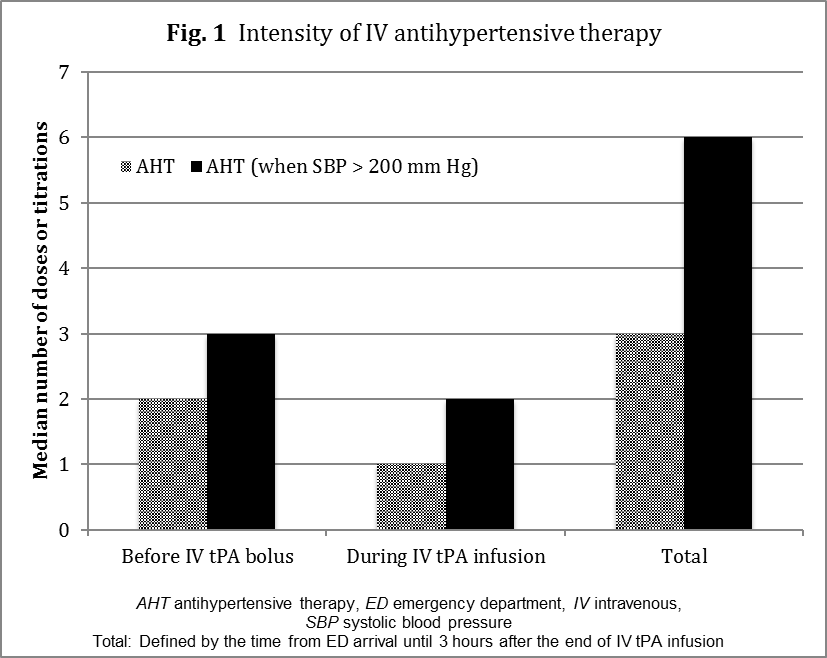
Figure 1: Intensity of IV antihypertensive therapy
The median number doses and titrations increased to 6 when documented SBP readings were greater than 200 mm Hg between ED arrival time and the end of the IV tPA infusion. Interestingly, 61% of the AHT Before group required more than 2 doses or titrations before the initial IV tPA bolus. That proportion increased to 91% when SBP reached 200 mm Hg during AIS management in the ED. Overall, the median time between the first dose of any IV antihypertensive medication and IV tPA was 25 minutes.
In subjects who required BP reduction prior to tPA, labetalol was initially the most common IV antihypertensive agent used (74%) followed by hydralazine (13%) and nicardipine (4%). A total of 22% of subjects required the continuous infusion of nicardipine before IV thrombolysis. That proportion increased to 35% when we included those subjects who also had nicardipine initiated during tPA infusion. Overall, 22% of subjects who received IV AHT before IV thrombolysis required more than one antihypertensive agent. That proportion increased to 48% when we included all AHT subjects from ED arrival time until the end of the tPA infusion. No cases of clinically significant hypotension were identified within the entire AHT group.
Discussion
In this study, as expected, subjects were mostly in their 60s with a history of hypertension with multiple risk factors for stroke. They were equally likely to be men or women, and Caucasian or non-white. All patient characteristics, hospital factors and standard of care determinants that could affect the timing of IV tPA administration were similar between the AHT and Control groups. More subjects had congestive heart failure within the Control group but we do not deem this finding to be clinically significant in the delivery of standard of care for AIS. This observation may simply be explained by the fact that congestive heart failure is more likely to result in longitudinal healthcare with pharmacotherapy than the other co-morbid conditions studied that might be more silent in nature (e.g. diabetes, hypertension), thereby lessening the likelihood of experiencing hypertensive emergencies.
To compare our cohort of patients, we measured the time of arrival to the start of multimodal CT/CTA/CTP since it was suggested as an important metrics for measuring quality of care [10]. There were no differences between the two study groups in terms of type of multimodal imaging studies and time to start multimodal CT or multimodal CT-to-needle time. Consequently, the conduct of imaging studies does not appear to affect potential differences in time delays between the groups. Although not considered a metrics for quality of care, timing in relation to the start of laboratory studies was also collected, and no differences were found. A very small but greater number of subjects required rapid sequence intubation in the ED in the AHT before subgroup (n=3 vs. 1). However, when further evaluating these four cases, they did not demonstrate longer delays in standard of care according to the documentation provided in the medical records. Thus, we considered these subjects for analysis since they represented less than 5% of the entire study cohort.
In terms of outcomes collected and compared, no differences were identified between the two cohorts with regards to discharge disposition, mortality, or complications. Of interest, the percentage of patients treated with IV thrombolysis who had a symptomatic intracranial hemorrhage within 36 hours of treatment, a core metric for quality of care, did not differ [10]. These outcome findings also support the comparability of both groups studied.
Eighteen percent of subjects required BP reduction for tPA eligibility which is somewhat similar to what had been previously reported in the NINDS trial and other reports [1, 3]. Many subjects required IV AHT during IV tPA infusion to maintain eligibility. Overall, 26% of subjects required BP control between ED arrival and the end of IV thrombolysis infusion. These additional cases of IV AHT during IV tPA infusion are rarely reported in stroke database as those mostly capture patients who only received IV AHT before the initial tPA bolus. Although clinicians may perceive severe hypertension as an important factor that can delay tPA administration, we measured its extent to be approximately 10 minutes in our cohort of subjects. We initially expected this difference to be greater and to reflect the median time between the first IV AHT given and the time of tPA administration, which was 25 minutes. This suggests that the administration of IV AHT is not the only factor that delays IV tPA administration when severe uncontrolled hypertension is present. Interestingly, Martin-Schild et al. [9] found a median difference of 20 minutes when they studied aggressive BP lowering therapy between two IV AHT regimens. We could not conduct a similar analysis since our number of subjects was smaller and the initial IV AHT was not standardized at our institution. Recently, an observational study by Skolarus et al. [3] reported an increase in DTT (mean of 10 minutes) among matched patients with prethrombotic AHT against a control group treated at community hospitals [3]. Although our methodology was more controlled for confounders, the results of our study are comparable with this report.
The clinical significance of a median DTT delay of 10 minutes may be perceived as modest in many medical conditions, but potentially important in AIS patients when quantifying neuronal damages and their associated neurological deficits [3,11,12]. We also speculate, based on our clinical and research observations, that a 10 minute delay could have potentially allowed more subjects to received IV tPA within AHA metrics for measure quality of care of 60 minute of door-to-needle time. A more standardized IV AHT approach could have potentially improved these metrics [10] in our study, particularly in subjects with recorded SBP greater than 200 mm Hg in whom the intensity of IV AHT was clinically considerable.
Until recently, limited data was known about the contemporary treatment of hypertensive emergencies in U.S. hospitals. The STAT registry was a U.S.-based, multicenter, observational, cross-sectional survey of management practices and outcomes for patients with acute severe hypertension treated with IV AHT. The results highlighted that IV AHT used varied considerably with 64% of patients requiring multiple IV antihypertensive agents acutely [13]. A subset analysis of the STAT registry including only patients with a primary neurologic diagnosis reported an association between mortality and lower minimum BP values during the acute phase of therapy. Despite this well-defined registry, only 5% of the initial cohort of subjects included AIS patients, the majority of patients having hemorrhagic stroke (87%) [14]. Clearly, little information describing the IV AHT in AIS is available in the literature and our study will contribute to advance our understanding of this clinical dilemma.
Similar to the findings of the STAT registry, our study revealed that the most common initial IV AHT was labetalol via boluses (74%). Approximately half of the patients (48%) in our study were treated with more than one IV antihypertensive agent. Even with the use of polypharmacy, the number of doses of any IV AHT boluses or dosing titrations ranged from 1 to 10. The use of polypharmacy with IV AHT in IV tPA eligible patients constitutes one of the most interesting findings in this study. We believe that the use of polypharmacy to achieve BP control in AIS may not be optimal, especially in patients with very severe uncontrolled hypertension. In fact, subjects who had a recorded SBP greater than 200 mm Hg were more likely to require IV AHT polypharmacy along with multiples boluses and titrations prior to and during the IV tPA infusion. AHT polypharmacy could be a source of medication errors in the ED considering the advanced age and comorbid conditions in most AIS patients.
Medication errors have been recognized in the setting of AIS. Patients with severe uncontrolled hypertension require advanced dosing decisions that must consider the patient’s condition as well as the various pharmacokinetic and pharmacodynamic properties of all antihypertensive agents available in order to prevent excessive BP reductions in the setting of a pressure-sensitive stroke [15]. In our study, this was reflected in a few isolated cases where we observed that SBP approached normotension during the acute management of AIS. We did not identify enough cases to further study this observation. In the STAT registry, 6.2% experienced iatrogenic hypotension requiring treatment with fluids and/or vasopressor therapy. We did not identify any of these above-mentioned scenarios in our study. Although no cases of hypotension requiring intervention were identified, the concept of normotension is not well defined in the setting of AIS. Some clinicians argue that although these cases did not require intervention, they could be considered as too aggressive for BP reduction, thereby mimicking a state of relative hypotension within the penumbra.
In the STAT registry, patients initially treated with nicardipine were the most likely to be managed with a single intravenous agent. This finding could not be verified since most patients initially received either labetalol or hydralazine. The fact that half of the subjects received more than one therapy underscores the lack of data and consensus on optimal pharmacologic management in the setting of AIS. Unlike the report by Martin-Schild, et al. [9], our study included those subjects treated between 3 and 4.5 hours, and any IV antihypertensive agents (in addition to labetalol and nicardipine). Although observational registry data like these add considerably to our understanding of acute BP management during stroke, future studies should focus on prospective data collection in an attempt to better define best practices in relation to quality of care metrics. This quality improvement exercise is not required by JCAHO, but it would be interesting to duplicate similar studies with other academic stroke centers and non-academic institutions. We believe our study supports the rationale for a standardized approach when managing BP prior to and during IV tPA administration. A similar multicenter analysis should be conducted to identify areas of improvement in stroke management when BP control must be addressed.
Limitations
The retrospective nature of this study is a methodological limitation but at the same time, it also captured the reality of the clinical dilemma of treating severe uncontrolled hypertension during AIS. Obviously, the exact time at which point SBP was less than 185 mm Hg could not be captured. By default, we had to assume that IV tPA was administered approximately at that same time. For the laboratory-to-needle time, we used the time when the blood samples was documented as received and not necessarily reported to the clinicians, although there is no reason to suspect that this fact would be any different between the two groups studied. Of interest, this study was conducted within an academic stroke program with comprehensive resources. One must consider that the reported results may not apply to a non-academic institution or to other academic stroke programs at large as their hospital resources might be different.
Our study is the first published analysis with a complete evaluation of BP control related to IV tPA administration that not only characterized IV AHT used, but also evaluated several confounders affecting DTT while adjusting for stroke-to-arrival time. Stroke-to-arrival time is a critical variable affecting DTT in observational stroke studies [8]. We also observed longer DTT among patients who presented early on after stroke onset. We believe our findings are likely to be found in other stroke centers in the U.S. In 2009, the AHA released updated stroke guidelines that extended the therapeutic window to 4.5 hours. Since our study period overlapped with this time period, this may have affected the number of patients that could have been included over a six-year period with the new updated guidelines. However, we do not believe this guidelines update introduced any biases between the two groups compared since the subjects recruited were almost equally distributed per year. Our study cannot account for the change in stroke personnel that occurred in over the study period however, as some clinicians might be more aggressive with IV AHT than others.
Conclusion
We identified IV AHT as a significant determinant that could delay the administration of IV tPA. We documented the need for polypharmacy and multiples doses or titrations to achieve and maintain tPA eligibility. Of interest, the intensity of IV AHT was significantly greater among patients with severe uncontrolled hypertension and SBP reaching greater than 200 mm Hg. Consequently, clinicians should anticipate more aggressive IV AHT initially so that IV thrombolysis is not further delayed by BP control in IV tPA candidates. We suggest that other clinicians report on this particular clinical dilemma to verify the extent of our findings in other academic and non-academic stroke centers.
Acknowledgements
The authors acknowledge the contribution of The Medicines Company for the resources and guidance provided, and The Medical University of South Carolina Center for Academic Excellence for the initial manuscript review. This project was supported in part by the South Carolina Clinical & Translational Research Institute, MUSC's CTSA, NIH/NCATS UL1 TR000062. We also acknowledge the contribution of Robert Helmer, PharmD and Jennifer Bekker, PharmD for their initial contribution in data collection.
Conflicts of Interest
The study was supported by a research grant from The Medicines Company to the Medical University of South Carolina. Data were collected, handled, and analyzed independent of the sponsor. The authors were solely responsible for the design and conduct of this study and the publication of its results. The sponsor was given an opportunity to review the final results prior to publication.
Dr. Lapointe is a consultant and a member of the speaker bureau for The Medicine Company. He was also a co-investigator in the STAT Registry and the Accelerate Trial. Drs. Ho and Nietert have no conflicts of interest to report.