Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
P. Sagadevan1, R. Deepalakshmi 1, DevarajBharathi2, M. Nagarajan1, M. Raguhnath1, P. Janarthanan3,
1PG department of biotechnology KSG College of Arts and Science, Coimbatore, Tamilnadu, India
2Department of Biotechnology, Hindustan college of Arts and Science, Coimbatore, Tamilnadu, India
3Aviagen, Tamilnadu, India
*Corresponding author: P. Sagadevan, PG department of biotechnology KSG College of Arts and Science, Coimbatore, Tamilnadu, India.
Received: June 14, 2021
Accepted: June 30, 2021
Published: July 05, 2021
Citation: P. Sagadevan, R. Deepalakshmi, DevarajBharathi, M. Nagarajan, M. Raguhnath, P. Janarthanan. “Green Blend of Silver Nanoparticles by Utilizing Andrographis Echioides Leaf Fluid Remove and Their Antibacterial Action”. J Pharmacy and Drug Innovations, 2(5); DOI: http;//doi.org/03.2020/1.1021.
Copyright: © 2021 P. Sagadevan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The current investigation reports an eco-accommodating, green union of silver nanoparticles (AgNPs) utilizing fluid leaf separate of Andrographis echioides. At first the blend of AgNPs was affirmed by visual perception as shading change. Further, the morphology of the biosynthesized nanoparticles, normal size and presence of basic silver were portrayed by UV-Visible spectroscopy, checking electron microscopy (SEM), energy dispersive X-beam (EDX) and fourier change infrared spectrometer (FTIR). Subjective phytochemical screening and FTIR otherworldly pinnacles upheld the part of phytochemicals in bark separate for the metal decrease, adjustment and covering of silver nanoparticles. XRD contemplates exhibited that translucent nature and their normal size of nanoparticles was 25 nm as controlled by utilizing Scherrer's equation. SEM pictures uncovered that the orchestrated AgNPs were circular fit as a fiddle. In vitro antibacterial impact of different convergence of AgNPs was examined against both Gram positive and Gram-negative bacterial strains. The outcome shows that biosynthesized AgNPs has huge antibacterial movement.
Introduction
Nanoscience and nanotechnology is an arising as a quickly developing zone with enormous application in biotechnology. These days, metal nanoparticles of silver and gold has been the subject of centered examination zone because of their special optical, mechanical and synthetic properties that are not the same as those enormous materials [1]. Among these metal nanoparticles, silver has become a focal point of interest since they assume a critical job in material and drug businesses [2].
Up until now, various methodologies are accessible for the amalgamation of silver nanoparticles (AgNPs) utilizing electrochemical [3], microwave helps measure [4], sono-synthetic [5], radiation help [6], invert micelles measure [7], stage move measure [8] and photochemical union [9]. A large portion of these strategies are veryexpensiveand likewise utilizes poisonous synthetic compounds which mayleadto possible natural and wellbeing hazards [10]. Lately, phytocompoundsfacilitatedsynthesis of AgNPs are picking up centrality due to their availabilityand eco-accommodating [11]. Biosynthesis of silver nanoparticles utilizing plants, forexample ,Eucalyptus chapmaniana[12], Momordicacharantia [13], Terminaliabellirica[14]and a lot more plants have been accounted for. Silver nanoparticles have various applications, for example, bio naming, fighting of organisms, discovery of malignancy, drug conveyance and different illnesses [15].
Andrographis echioidesis a conventional Indian restorative plant and have a place with the family ofAcanthaceae. Andrographisgenus is getting expanded consideration as it is utilized in Indian conventional prescriptions like Ayurveda and Unani. In any case, until now, less consideration has been paid to combination nanoparticles from this plant. Consequently the current examination aimed to biosynthesis of AgNPs from leaf remove ofA. echioodes. We have exhibited the phytochemical screening, biosynthesis, portrayal of silver nanoparticles and their antibacterial exercises.
Materials and methods
Chemicals and reagents
Silver nitrate (AgNO3), methanol, 2, 2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid was purchased from Sigma Aldrich. All other reagents and chemicals used in this research were of analytical grade.
Plant collection and authentication
The leaf samples of Andrographis echioides were collected from Papampatti village, Coimbatore. The collected plant samples was identified and authenticated by BSI (Reference number: BSI /SRC/5/23/2019 tech\245).
Preparation of leaf extract
The leaves was washed thoroughly thrice with distilled water and dried for 7 days. The fine powder was obtained from stem bark using kitchen blender. About 10 g of leaf powder were extracted with 100 mL of double distilled water using soxhlet extractor. After completion of the extraction, concentrated, dried and kept at 4˚C until further use.
Qualitative Phytochemical screening
Phytochemical screening of leaves of Andrographise chioideswere performed for the qualitative detection of different phytocompounds such as alkaloids, flavonoids, saponins, glycosidas and tannins. All phytochemical tests were performed according to the standard methods described by Al-Owaisi[16], Sheel [17].
Biosynthesis of silver nanoparticles
Silver nitrate solution (1mM) was prepared in amber bottle and stored at dark place. The prepared leaf extract was mixed with 1mM AgNO3 solution in 1:9 proportions and kept at room temperature for 10-20min.PurifiedAgNPs from the samples were obtained by centrifuging the solution at 10,000 rpm for 20 min. The precipitate was re-dispensed with 10 ml double distilled water and again centrifuged at 10,000 rpm for 10 min for removing excess biomass. The pellet of Ag nanoparticles were collected carefully and dried 70 °C for 12 hours. The dried powder of AgNPs was used for further test.
Characterization of AgNPs
UV-Visible spectroscopy
The formation of nanoparticles was preliminarily confirmed by visual observation as colour change from watery to brown colour. Further the reduction of Ag ions in the solution mixture was confirmed by UV-Visible spectroscopy. The spectral data of synthesized AgNPs were recorded from 300 - 700 nm at a resolution of 1 nm.
SEM and EDX
The morphology of AgNPs was determined by SEM (Hitachi S -340N). SEM samples were prepared on a carbon coated copper grid by placing a small amount of AgNPs on the SEM grid, excesssamples was cleaned by blotting paper and then samples were allowed to dry under mercury lamp. This experiment was conducted at an accelerator voltage of 20 kV. Energy dispersive X-ray analysis was also taken on the same instrument.
XRD analysis
The structure of the AgNPs was determined using XRD technique. For X-ray diffraction studies (XRD), dried silver nanoparticles were coated on XRD grid (XPERT - PRO) and diffraction recorded in the 2θ range from 0˚ to 80˚. The spectral data was operated at 40 kV and a current of 40 mA.
FTIR
The biologically active components that are responsible for silver reduction, formation and capping were identified using Fourier Transform Infra-Red (FTIR) spectroscopy (Nicolet is5, Thermo Scientific) by KBr pellet method. FTIR spectral reading was carried out in the range from 500 to 4000 cm-1 at a resolution of 4 cm-1.
Antibacterial activity
The antibacterial activity of AgNPs were carried out by agar well diffusion method described by Gudikandula [28] against E.coli and S, aureus. Muller-Hinton Agar (Himedia, Mumbai) plates were prepared, sterilized and solidified. Each bacterial strain (1 × 105CFU/mL) was swabbed uniformly on the prepared individual petri plates using sterile cotton swabs. Each well of size approximately 6 mm are made on prepared plates using gel puncture. Different concentration of AgNPs (10, 20, 30 and 40 µL) was loaded into the wells of all plates. After incubation for 24 h at 37˚C, the plates were examined for zone of incubation in mm.
Results and discussion
The consequences of subjective phytochemical screening of leaf concentrate of Andrographise chioides were appeared in Table 1. Phytochemical screening demonstrated the presence of saponis, alkaloids, glycosides, tannins favonoids and terpenoids. It was accounted for that the leaves of leaf concentrate of Andrographis echioides contain steroids, phenols, flavonoids, saponins, terpinoids. Presence of these naturally dynamic mixes may assume a huge job in arrangement, covering and adjustment of AgNPs.
|
S.No |
Phytochemicals compound |
Aqueous Extraction of Andrographisechioides |
|
1 |
Alkaloids |
+ |
|
2 |
Flavonoids |
- |
|
3 |
Saponins |
+ |
|
4 |
Tannins |
+ |
|
5 |
Triterpinoids |
+ |
|
6 |
Glycosides |
+ |
Table1: Preliminary Phytochemical analysis Andrographis echioides leaf
Biosynthesis of AgNPs
For the biosynthesis, bark separate was blended in the watery arrangement of AgNO3 at room temperature. At first the shading changed from watery to brown tone (Fig. 1) inside 20 minutes after which no shading change was noticed. The divert in shading from watery to brown tone shows the arrangement of AgNPs and this change might be because of the SPR (Surface Plasmon Resonance) of AgNPs in the response tests.

Fig. 1: Colourization images of silver nitrate, plant extract, and Ag NPs
The arrangement of silver nanoparticles was estimated utilizing UV-Visible Spectroscopy from 300-700 nm frequency range and the most elevated ghostly pinnacle was seen at 454 nm (Fig. 2). It was accounted for before that the absorbance around 450 nm for Ag as a trademark highlight of metal nanoparticles.
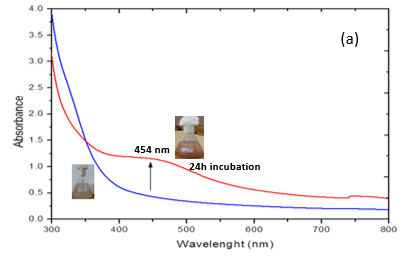
Fig. 2: UV-visible analysis of silver nanoparticles
SEM picture (Fig. 3) indicated the morphology of AgNPs is prevalently round shape with conglomeration. SEM pictures indicated the size of individual AgNPs were gone from 10 to 90 nm.Similar to our examination, Krishnaraj [18] integrated the silver nanoparticles from Bacobamonnieri concentrate and they got changing size and morphology of the silver nanoparticles ran between 2 to 50 nm from TEM investigation.

Fig. 3: SEM images of Silver nanoparticles
EDX range from Fig. 4 speaks to flag from Ag (0.3 and 3 keV) along with O (0.5 keV). The range at 3 keV demonstrates that the Ag has been effectively recognized. For the most part, Ag nanoparticles show run of the mill optical top at 3 keV. The ingestion pinnacle of O in EDX range may be because of the contribution of phytocompounds in covering and balancing out of Ag nanoparticles through O related gatherings.
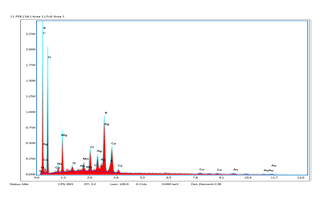
Fig. 4: EDS analysis of silver nanoparticles
The XRD designs appeared in Fig. 5 exhibit the translucent idea of integrated AgNPs. Most noteworthy 2θ diffraction tops saw at 38.4˚, 47.1˚, 76.1˚ and 78.2˚ which comparing to (111), (200), (220), (311) and (420) planes separately, which affirms the face focused cubic structure of integrated nanoparticles [19]. Comparing planes was coordinated with JCPDS, File No. 4-0783 (Joint Committee on Powder Diffraction Standards) values for silver. The normal mean size of biosynthesized nanoparticles were resolved utilizing Debye - Scherrer's recipe, D=0.9λ/β cosθ and is assessed to be around 25 nm from the expansiveness of the reflection. Where, λ is the frequency of X-beams utilized, β is the widening of diffraction line utilized estimated at half greatest force and θ is Bragg's diffraction point. The additional diffraction tops (*) in the XRD example might be because of the crystallization of bio-natural stage in the plant remove.
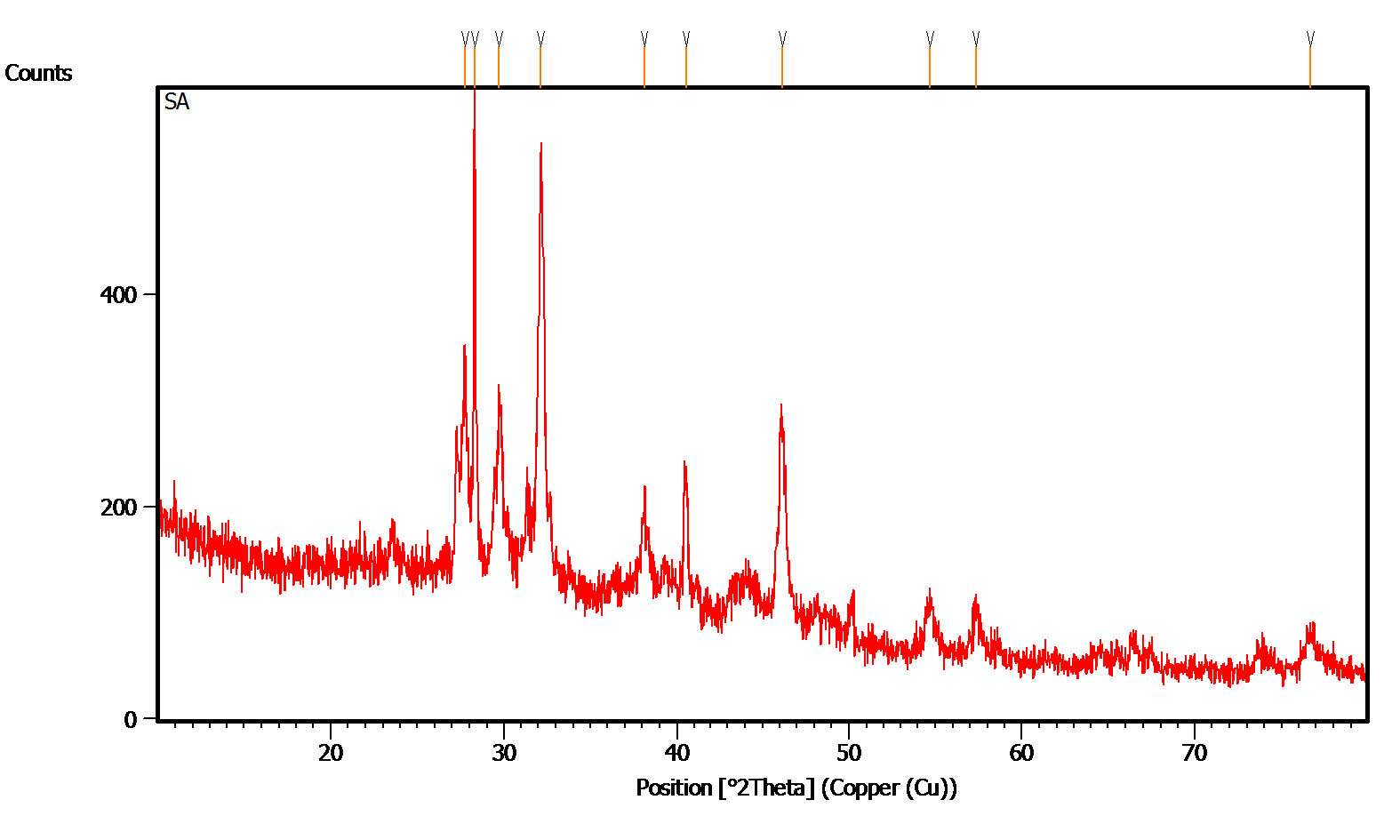
Fig. 5: XRD analysis of silver nanoparticles
FTIR spectra in Fig. 6 showed the presence of phytochemicals which might be answerable for the blend of AgNPs. The most noteworthy top at 3364.92, 2978.32 and 1080.30 cm-1 credited to N-H stretch, C-H stretch vibration of amides, alkanes and aliphatic amines. The medium groups around3278-3116 3cm-1 compared to O-H stretch and C–H stretch conceivable of liquor, phenols and amines. Different tops at 2360, 21583, 1921cm-1 were allocated to the presence of aldehydes (H-C=O, C-H), nitrogen (N-O) and sweet-smelling mixes (C-H), individually. The tops around the 1000-500cm-1may be expected the presence of OH gatherings. This spectroscopic examination affirmed the presence of amides, phenol, nitrogen and sweet-smelling mixes has a solid restricting liking with Ag and furthermore assume a critical part in diminishing and covering of Ag particles for the change of Ag+ to AgNPs.

Fig. 6: FTIR analysis of silver nanoparticles
Antibacterial action
The integrated AgNPs indicated huge enemy of bacterial action against both gram positive and gram-negative microbes. The antibacterial impact of different centralization of AgNPs (10 µL) was appeared in Fig. 7. The most elevated enemy of bacterial zone of restraint was recorded in by E. coli, followedby S.aureus. From this examination, AgNPs demonstrated high zone of restraint against Gram negative bacterial strains contrasted and Gram-positive microorganisms. This might be because of the presence of dainty peptidoglycan layer in the Gram-negative microbes yet in the event of Gram-positive microscopic organisms, which are comprised of thick peptidoglycan layer. It was additionally announced that the charged AgNPs can tie adversely charged Gram negative bacterial cell divider hitter on opposite [20,21].

Fig. 7: Antibacterial activity of silver nanoparticles
Conclusions
In this investigation, we have combined AgNPs by eco-accommodating and savvy technique utilizing Andrographis echioides leaf extract. The phytochemicals present in the stem bark concentrate of plant go about as a solid decreasing and covering specialists for the arrangement of AgNPs. The morphology and size of the silver nanoparticles were described by UV-Visible spectroscopy, SEM, EDX and XRD reports uncovered that incorporated silver nanoparticles were glasslike in nature with normal mean size as 25 nm and FTIR examines upheld the affirmation of bioactive mixes for the Ag+ decrease. Biosynthesized AgNPs indicated significant antibacterial action against Gram negative microbes contrasted with Gram positive microscopic organisms. Hence, as-blended Ag NPs can be utilized as biocidal specialists.
Irreconcilable circumstance
The creators have proclaimed no irreconcilable circumstance