Adel E. Ibrahim1, Magda Elhenawee2, Hanaa Saleh2, Mahmoud M. Sebaiy3*
1Department of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Port Said University, Port Said, 42511, Egypt.
2Department of Analytical Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt.
3Department of Medicinal Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, 44519, Egypt.
*Corresponding author: Mahmoud M. Sebaiy, Department of Medicinal Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, 44519, Egypt.
Received: April 03, 2021
Accepted: April 16, 2021
Published: April 20, 2021
Citation: Adel E. Ibrahim, Magda Elhenawee, Hanaa Saleh, Mahmoud M. Sebaiy. “Overview on Hepatitis C, Treatment Strategy, Instrumental Analysis of Anti-HCV drugs’’. J Pharmacy and Drug Innovations, 2(2); DOI: http;//doi.org/03.2020/1.1014.
Copyright: © 2021 Mahmoud M. Sebaiy. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This literature review is dealing with Hepatitis C, treatment strategy, and different analytical techniques for determination of recently used anti-HCV drugs specifically Sofosbuvir, Simeprevir, Ledipasvir, Daclatasvir and Velpatasvir in different pharmaceutical and biological samples.
Hepatitis C
Liver is a very vital organ responsible for many important processes inside the body. Such processes include digestion of food, metabolism of carbohydrates, fats and proteins, storage of glucose, iron and vitamins (A, D, E, K and B12), production of some plasma proteins, regulating blood clotting, detoxification of blood from harmful substances (eg. Alcohol and drugs), immunity by producing immune factors and removing bacteria from blood stream……..etc [1, 2]. Hepatitis is defined as the inflammation of liver. Toxins, heavy alcohol consumption and even some medications can cause hepatitis. The most common cause for hepatitis is viral infection with viral Hepatitis A, Hepatitis B and Hepatitis C viruses. Hepatitis A is spread via foecal-oral route, while Hepatitis B and C are spread via contaminated blood and blood products. Viral Hepatitis A and B can be prevented by immunization. Acute hepatitis A and B infections resolve without treatment in the majority of adults. Medications are mostly given to relieve their symptoms [3].
Hepatitis C virus (HCV) is the most dangerous type of this group of viruses. HCV was first discovered in 1989 and is now affecting more than 170 million people worldwide [4]. Since then a lot of research had been made on HCV which led to decrease in its transmission. The highest prevalence is found in North Africa, Middle East and central Asia with mortality rate around 350 000 deaths per year [5].
Hepatitis C is called a silent disease because people can be infected without knowing it. Also symptoms from acute infections can include fever, gastric upset and feeling fatigue which are common with many other diseases. Only 15-25% of acute infections can be self limiting, while more than 80% develop chronic HCV infections which can take decades to develop its symptoms. But when symptoms appear, they are often indicating advanced liver disease. Chronic HCV infections are serious and can lead to liver damage, cirrhosis, liver failure and carcinoma [6].Acute HCV infections are asymptomatic and about 15-20% of patients can clear acute infections, while the remaining majority develops into chronic infections [7]. Chronic HCV infection affects liver cells leading to cirrhosis, end-stage liver disease, and hepatic cancer. Chronic HCV infections caused about 350000 deaths per year [8].
HCV is a single-stranded RNA virus. A single HCV polyprotein is translated, and then cleaved by cellular and viral proteases into three structural proteins (core, E1, and E2) and seven non-structural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B) [9]. There are at least 6 genotypes of HCV that have arisen from the fast viral replication enabling it to adapt to host immune response and antiviral drugs. Genotyping is used as treatment guide since some viral genotypes respond better than others to different therapies.
HCV transmission is mainly by blood; however rare cases are transmitted sexually especially when associated with human Immunodeficiency virus (HIV) or on having relationships with multiple partners. Blood transfusion and reuse of injection needles are the major transmission routes globally. Mother to child transmission during pregnancy occurs in 2-8% from infected mothers [10]. Sexual transmission of HCV to partner is very rare [11].
HCV diagnosis relies on HCV antibodies detection and tests that detect HCV RNA. Presence of HCV antibodies without detection of viral RNA resolved or treated infections, but detecting both simultaneously indicates current infection. HCV is a single stranded RNA virus. HCV single poly-protein of 3011 amino acids is translated then cleaved by viral and cellular proteases into three structural proteins and seven non-structural proteins that are (P7, NS2, NS3, NS4A, NS4B, NS5A and NS5B) [9].
Seven genotypes of HCV were discovered. Genotype 1 is the most common worldwide genotype in about 46.2% of HCV infected population. Genotype 2 occurs to about 9.1% of HCV infections and was found to be the easiest for treatment. Genotype 3 occurs to about 30% of infected population globally and is the most difficult genotype in treatment. Genotype 4 is confined mostly to Africa. Egypt is considered the highest in prevalence of HCV in the world and 90% of infected Egyptians are infected with genotype 4. Genotypes 5, 6 and 7 occur in smaller prevalence [12].
HCV treatment
Early treatment of HCV was found to be much more effective than late stage treatments. Milestones in HCV treatment are summarized in (Figures 1 [13] and 2 [14]). In 1991, FDA approved the first interferon (Intron A) for treatment of HCV. Ribavirin was then approved in 1998 to be used in combination with interferon for treatment [15]. The leap forward in HCV treatment wasn’t until the discovery of directly acting antiviral drugs (DAADs).
In 2011, FDA approved triple therapy using two DAADs, boceprevir and telaprevir, together with pegylated interferon and ribavirin. Unfortunately, the safety profile of this treatment was poor. In 2013, FDA approved three DAADs; Simeprevir (SMP) as new NS3 protease inhibitor, Sofosbuvir (SFS) as NS5B RNA polymerase inhibitor and daclatasvir (DAC) as NS5A inhibitor. These drugs were approved as combination therapy with peg-interferon and ribavirin for 12-24 weeks [10]. Ledipasvir (LDS) and velpatasvir (VLP) were approved as NS5A inhibitors in 2014 and 2015, respectively. It wasn’t until late 2014 when interferon free regimen was approved.
The new regimens comprise a NS5B nucleoside inhibitor (eg. SFS) together with a protease inhibitor (eg.SMP) or NS5A inhibitor (eg.LDS, DAC, VLP), with or without ribavirin. The first approved combined single dose tablet was Harvoni® (SFS and LDS) then in 2016 Epclusa® (SFS plus VLP) was approved by FDA.
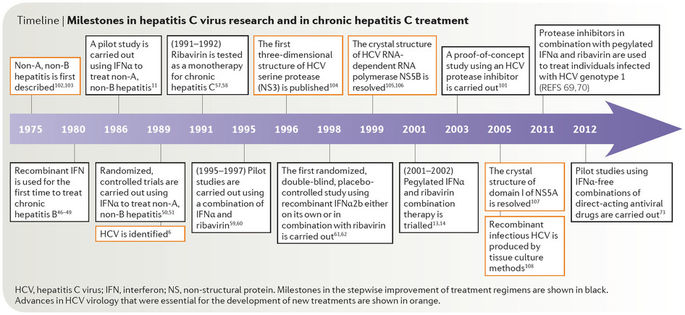
Figure 1: Milestones in HCV treatment.
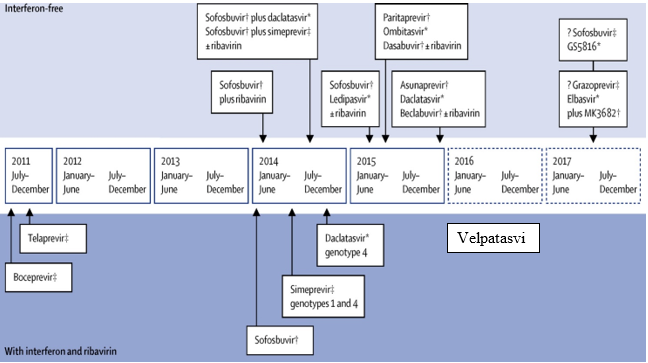
Figure 2: DAADs discovery milestone
Sofosbuvir (SFS) [16, 17]
* Molecular formula: C22H29FN3O9P.
* Molecular weight: 529.453 g/mol.
*IUPAC name: propan-2-yl (2S)-2-[[[(2R,3R,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyloxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate (Figure 3).
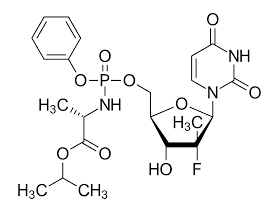
Figure 3: Sofosbuvir chemical structure
Physical properties:
SFS is white to off-white crystalline powder which is slightly soluble in water and soluble in methanol. It has pKa values of 9.38 and 10.30.
*Mechanism of action:
SFS is a prodrug which is metabolized by body into its active form that act as defective nucleotide analog for HCV NS5B (non-structural protein 5B) RNA-dependent RNA polymerase. SFS prevents HCV viral replication by binding to HCV NS5B polymerase's active site and preventing further replication of HCV genetic material. After oral administration of the drug, SFS reaches its maximum plasma concentration in about 0.5 to 2 hours with a maximal concentration (Cmax) of 567ng/mL.
*Literature review:
Reviewing literature revealed several methods for determination of SFS in pharmaceutical products and in
plasma using liquid chromatography Table 1 summarizes LC methods for determination of SFS.
|
Matrix |
Column |
Mobile phase |
System |
Ref. No
|
|
Plasma |
C18 |
0.1% formic acid: ACN (50:50) |
UPLC-MS/MS |
[18] |
|
Plasma |
C18 |
0.1% formic acid: ACN (gradiant) |
UPLC-MS/MS |
[19] |
|
Tablets |
C18 |
Water:MeOH (70:30) |
HPLC-MS/MS |
[20] |
|
Plasma |
C18 |
0.5% formic acid: MeOH (30:70) |
UPLC-MS/MS |
[21] |
|
Plasma |
C18 |
0.1% Phosphoric acid: ACN (68:32) |
HPLC-UV 228nm |
[22] |
|
Tablets |
C18 |
Phosphate buffer pH 4.0: MeOH (50:50) |
HPLC-UV 262nm |
[23] |
|
Tablets |
C18 |
0.1% formic acid: ACN (60:40) |
HPLC-UV 260nm |
[24] |
|
Tablets |
C18 |
MeOH: ACN (90:10) |
HPLC-UV 260nm |
[25] |
|
Tablets |
C18 |
0.2% Phosphoric acid: ACN (gradiant) |
UPLC-UV & MS/MS |
[26] |
|
Serum |
C18 |
0.05% Phosphoric acid: ACN (gradiant) |
HPLC-UV |
[27] |
Table 1Table 1: Summary of reported LC methods for SFS determination
Other spectrophotometric methods were published [24, 25, 28]. The spectrophotometric methods were done at wavelength 260nm using methanol as organic solvent. The limits of quantitation for the reported spectrophotometric methods were higher than 2.5µg/mL and the determination ranges started from 5-100µg/mL .
Only one HPTLC and CE method for SFS determination was reported [24]. HPTLC method used range of 25-100µg/mL and had the lowest sensitivity that LOQ was 7µg/mL. The highest sensitivity was for CE method and LOQ was 0.3µg/mL
* Molecular formula: C38H47N5O7S2.
* Molecular weight: 749.942 g/mol.
*IUPAC name: (1R,4R,6S,7Z,15R,17R)-N-(cyclopropanesulfonyl)-2-hydroxy-17-([7-methoxy-8-methyl-2-[4-(propan-2-yl)-1,3-thiazol-2-yl]quinolin-4-yl]oxy)-13-methyl-14-oxo-3,13-diazatricyclo[13.3.0.0] octadeca-2,7-diene-4-carboximidic acid (Figure 4).
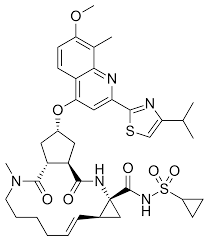
Figure 4: Simeprevir chemical structure
* Physical properties:
SMP is white to almost-white powder insoluble in water and slightly soluble in acetone and freely soluble in dichloromethane. It has pKa valueof 5.87. The drug is pharmaceutically available as water soluble sodium salt.
* Mechanism of action:
SMP inhibits HCV polyprotein cleavage via induced-fit binding to an NS3 catalytic site. After oral administration of the drug, SMP reaches its maximum plasma concentration in about 4 to 6 hours.
* Literature review:
Reviewing literature, A spectrophotometric method for determination of SMP in pharmaceutical dosage forms was published [29]. SMP was determined in presence of its oxidative degradation product at the range of 2.5-403µg/mL using different apectrophotometricmethods. Other LC determinations of SMP are summarized in Table 2.
|
Matrix |
Column |
Mobile phase |
System |
Ref. No. |
|
Plasma |
C18 |
Phosphate buffer: ACN (30:70) |
HPLC-UV |
[30] |
|
Tablets |
C18 |
ACN |
HPLC-UV |
[31] |
|
Plasma |
C18 |
AmmoniumFormate: ACN: MeOH (gradiant) |
HPLC-MS/MS |
[32] |
Table 2: Summary of reported LC methods for SMP determination
* Molecular formula: C49H54N8O6F2.
* Molecular weight: 889.0 g/mol.
*IUPAC name: methyl [(2S)-1-[(6S)-6-[4-(9,9-difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-[(methoxycarbonyl)amino]-3-methylbutanoyl]-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-5-yl]-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl]-3-methyl-1-oxobutan-2-yl]carbamate (Figure 5).
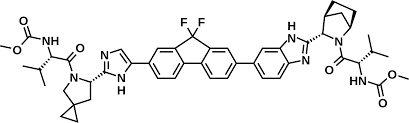
Figure 5: Ledipasvir chemical structure
* Physical properties:
LDS is white to beige powder. LDS is Insoluble in water, but soluble in acetonitrile and in methanol. It has pKa values of 4.0 and 5.0. The drug is pharmaceutically available as water soluble acetone solvate and Ledipasvircopovidone mixture.
*Mechanism of action:
LDS prevent hyperphosphorylation of NS5A polymerase which is required for viral protein production. It was found to be effective against HCV genotypes (1, 4, and 5) and with a lesser activity against genotypes (2 and 3). After oral administration of the drug, LDS reaches its maximum plasma concentration in about 4 to 4.5 hours with Cmaxof 323 ng/mL.
*Literature review:
Only two papers described the determination of LDS alone in tablet dosage forms [33, 34]. However; being only formulated in combination with sofosbuvir, several papers described TLC [35], spectrophotometric [36-40] and spectrofluorimetric [41, 42] besides chromatographic determinations of both drugs in several matrices. Table 3 summerizes some LC methods published for simultaneous determination of SFS and LDS.
|
Matrix |
Column |
Mobile phase |
System |
Ref. No. |
|
Plasma |
C18 |
0.1% formic acid: ACN (gradiant) |
UPLC-MS/MS |
[43] |
|
Plasma |
C18 |
0.1% formic acid: ACN (50:50) |
UPLC-MS/MS |
[44] |
|
Plasma |
C8 |
Ammonium formatebuffer:ACN:MeOH (gradiant) |
HPLC-MS/MS |
[45] |
|
Plasma |
C18 |
0.1% formic acid in MeOH:ACN: Acetic acid (63:25:12) |
HPLC-MS/MS |
[46] |
|
Tablet |
C18 |
Ammonium acetate buffer: ACN (35:65) |
HPLC-UV 245nm |
[47] |
|
Tablets |
C18 |
Phosphate buffer : ACN (50:50) |
HPLC-UV 254nm |
[48] |
|
Tablets |
C18 |
Trifluoroacetic in water: ACN:MeOH (30:50:20) |
HPLC-UV 267nm |
[49] |
Table 3: Summary of reported LC methods for LDS determination
* Molecular formula: C40H50N8O6.
* Molecular weight: 738.89 g/mol.
* IUPAC name: Dimethyl N,N'-([1,1'-biphenyl]-4,4'-diylbis[1H-imidazole-5,2-diyl-[(2S)-pyrrolidine-2,1-diyl][(2S)-3-methyl-1-oxobutane-1,2-diyl]])dicarbamate (Figure 6).
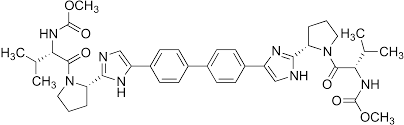
Figure 6: Daclatasvir chemical structure
*Physical properties:
DAC is white powder freely soluble in water. It has pKa values of 6.0 and 11.1. The drug is pharmaceutically available as dihydrochloride salt.
*Mechanism of action:
DAC inhibits HCV viral RNA replication and protein translation by directly inhibiting HCV protein NS5A polymerase. NS5A is critical for HCV viral transcription and translation, and as of 2014 it appeared that resistance can arise to daclatasvir fairly swiftly
After oral administration of the drug, DAC reaches its maximum plasma concentration in about 2 hours with bioavailability of 67%.
*Literature review:
A spectrophotometric and another spectrofluorimetric methods were published for determination of DAC [50, 51]. The spectrophotometric method used methanol as solvent for quantitation of DAC in range of 2-12µg/mL at wavelength 317nm. The spectrofluorimetric method was for simultaneous determination of DAC in presence of LDS.
Other LC methods for determination of DAC were reported either alone or in combination with sofosbuvir. Table 4 summarizes those reported chromatographic determinations.
|
Matrix |
Column |
Mobile phase |
System |
Ref. No. |
|
Plasma |
C18 |
Ammonium formate buffer: ACN (50:50) |
UPLC-MS/MS |
[52] |
|
Tablets |
C8 |
Phosphate buffer: ACN (75:25) |
HPLC-UV 306nm |
[53] |
|
powder |
Amylose |
ACN:MeOH containing diethylamine (gradiant) |
HPLC-UV 315nm |
[54] |
|
Tablets |
C18 |
Phosphate buffer: ACN (60:40) |
HPLC-UV 315nm |
[55] |
|
Plasma |
C18 |
Ammonium acetate buffer: ACN (56:44) |
HPLC-UV 318nm |
[56] |
|
Plasma |
C18 |
0.1% formic acid: ACN |
UPLC-MS/MS |
[57] |
|
Plasma |
C18 |
Ammonium formate buffer: ACN (50:50) |
UPLC-MS/MS |
[58] |
|
Tablets |
C18 |
Phosphate buffer: ACN (60:40) |
HPLC-UV 265nm |
[59] |
|
Tablets |
C18 |
Phosphate buffer: ACN (50:50) |
HPLC-UV 320nm |
[60] |
Table 4: Summary of reported LC methods for DAC determination
Velpatasvir (VLP) [16, 17]
* Molecular formula: C49H54N8O8.
* Molecular weight: 883.019 g/mol.
*IUPAC name: Methyl [(2S)-1-[(2S,5S)-2-(9-[2-[(2S,4S)-1-[(2R)-2-[(methoxycarbonyl)amino]-2-phenylacetyl]-4-(methoxymethyl)-2-pyrrolidinyl]-1H-imidazol-4-yl]-1,11-dihydroisochromeno[4',3':6,7] naphtho[1,2-d]imidazol-2-yl)-5-methyl-1-pyrrolidinyl]-3-methyl-1-oxo-2-butanyl]carbamate (Figure 7).
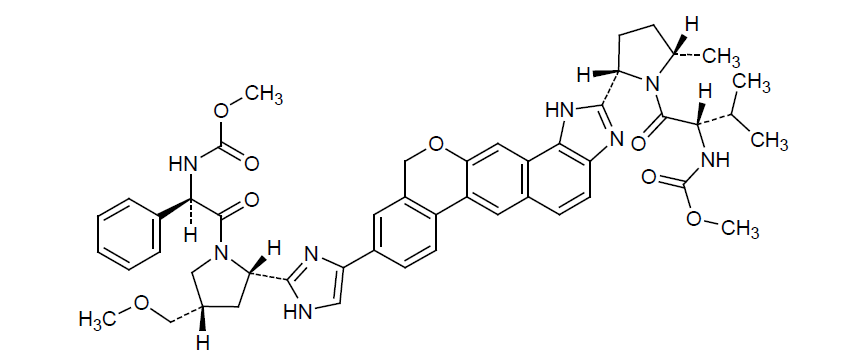
Figure 7: Velpatasvir chemical structure
*Physical properties:
VLP is off-white to yellow color powder insoluble in water and slightly soluble in methanol. It has pKa values of 3.8 and 6.0. The drug is pharmaceutically available as VLP copovidone mixture.
*Mechanism of action:
VLP is non-structural protein NS5A polymerase inhibitor. It inhibits HCV viral RNA replication and protein translation. After oral administration, VLP is 99.5% bound to plasma proteins with bioavailability of 25-30%.
*Literature review:
Only two LC methods were reported for determination of VLP in tablet dosage forms as listed in Table 5.
|
Matrix |
Column |
Mobile phase |
System |
Ref. No. |
|
Tablets |
C18 |
0.1% phosphoric acid in water:ACN (45:55) |
HPLC-UV 240 nm |
[61] |
|
Tablets |
C18 |
0.1% phosphoric acid in water:ACN (45:55) |
HPLC-UV 260 nm |
[62] |
Table 5: Summary of reported LC methods for VLP determination
Although some papers described validated methods for simultaneous determination of two DAADs (e.g. SFS/LDS, SFS/VLP, SFS/DAC), only two LC methods were developed for the quantification of some of the new DAADs using UHPLC-MS/MS [63, 64].
Conclusion:
This literature review is introducing brief summary about Hepatitis C disease, its treatment strategy, and instrumental analysis of recently used anti-HCV drugs specifically Sofosbuvir, Simeprevir, Ledipasvir, Daclatasvir and Velpatasvir in different matrices.