Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
Mohammed Abdulazim
Sulaiman AlRajhi University, Al Bukairiyah, Kingdom of Saudi Arabia
*Corresponding author: Mohammed Abdulazim, Sulaiman AlRajhi University, Al Bukairiyah, Kingdom of Saudi Arabia
Received: March 21, 2021
Accepted: April 16, 2021
Published: April 25, 2021
Citation: Mohammed Abdulazim. “Current knowledge on recurrent implantation failure in patients undergoing IVF: a narrative review’’. J Pharmacy and Drug Innovations, 2(2); DOI: http;//doi.org/03.2020/1.1012.
Copyright: © 2020 Mohammed Abdulazim. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Recurrent implantation failure (RIF) is an ill-defined, uncommon clinical entity diagnosed when there is failure to achieve pregnancy with repetitive embryo transfers [1]. Whereas the definition of recurrent implantation failure (RIF) is debatable, a recent study recommended defining RIF as the failure of implantation in three or more consecutive attempts of IVF, in which each cycle at least transfers 1-2 high quality embryos [1].
Introduction and background:
Recurrent implantation failure (RIF) is an ill-defined, uncommon clinical entity diagnosed when there is failure to achieve pregnancy with repetitive embryo transfers [1]. Whereas the definition of recurrent implantation failure (RIF) is debatable, a recent study recommended defining RIF as the failure of implantation in three or more consecutive attempts of IVF, in which each cycle at least transfers 1-2 high quality embryos [1]. Other studies have considered four as the cut-off for defining RIF, and many propose other cutoffs considering maternal age and known euploidy, with thoughts of restating the definition to two or more failed embryo transfers in appropriate populations. Incidence and prevalence of RIF are not commonly reported, due to scarce data that accurate represents them [2]. There are a number of risk factors for RIF. These include, but are not limited to, advanced maternal age, parental smoking, increased body mass index, and stress. The causal factors of failure of embryo implanting to the endometrium are typically uterine, paternal, or embryo related, or the type of IVF protocol used. Embryo implantation is a carefully regulated event, relying on multicomponent, bidirectional signaling between the embryo and endometrium [3]. This dual-directional finely tuned inter-reaction between endometrium and embryo that ultimately leads to apposition, attachment and invasion of embryos is obligatory for success of implantation and subsequent normal pregnancy events [4]. These processes are subject to thorough exploration and seem to include a list of mediators originating from the embryo, the endometrium, and the maternal immunologic system [5]. Even with the recent, huge advancement of IVF and its utility in achieving better pregnancy outcomes, the rate of implantation failure is still relatively high [6]. With the endometrium representing an important part of the implantation process, causes of endometrial dysfunction are first suspected when poor quality of transferred embryo is excluded as the cause of RIF [1]. The purpose of this review is to shade light on the recent updates on RIF in women undergoing IVF. This includes a short review of the culprit etiologies, clinical assessment, diagnosis, management approaches, and future directions related to RIF.
In Vitro Fertilization (IVF) is a procedure in which the fully mature oocyte is removed surgically from the ovary and is then fertilized with a sperm in a liquid in the laboratory. IVF over the past
25 years has been improved substantially in terms of success rate and complications, and now over 2 million babies have been born through this technique [7]. Indications of IVF in female infertility include irreparable tubal disease, women who have been previously sterilized by tubal blockade and they desire tubal recanalization but have poor prognosis, ovarian failure or diminished ovarian reserve (using donor oocyte), müllerian agenesis (through implanting the oocyte derived from the women in a uterus of a surrogate), severe intrauterine adhesions, and women carrying genetic disorders. Male factor infertility. Indications of IVF in males are unsuccessful IUI, unexplained infertility, and a sperm count of < 5 million/ml [8].
There are three protocols for IVF: Natural cycle, long protocol, short protocol. Natural cycle IVF involves the retrieval of an egg from the ovary without pharmacologically stimulating it, and mainly relying on the natural cycle of the woman. It is used in cases of suboptimal ovarian reserve, poor responders to ovarian stimulation, or those with fairly high risk of ovarian hyperstimulation syndrome (OHSS) when giving medications. Long protocol (known as the agonist cycles) is the most commonly and widely used around the world. In this protocol, GnRH agonists such as Buserelin or Leuprorelin, are used daily in a continuously fashion in order to eventually and indirectly cause downregulation of gonadotrophins production from the pituitary gland. This leads to a reduction in the stimulation of the ovaries and a reduction of serum estrogen to menopausal levels within 3 weeks. After suppression of the ovarian function, it can be stimulated by exogenous follicle stimulation hormone and human menopausal gonadotrophins injections until an adequate ovarian response is gained (check ovarian response below). The short protocol (known as the antagonist cycles) has a more immediate effect as compared to the long protocol for achieving menopausal levels of gonadotrophins. GnRH antagonists such as ganirelix is given in a daily 0.25 mg dose and are started on the 5th day of stimulation or once the lead follicle reaches a size of 1.4 cm on US. The doses are continued along with gonadotrophins until an adequate ovarian response is achieved [9].
After achieving sufficient ovarian response, hCG with a dose of 250 ug (recombinant hCG) or 10000 (urinary hCG), is used to provide the full maturation of the oocytes prior to retrieval. hCG is started 35 hours prior to retrieval when the lead follicle reaches 18 mm. Alternatively, GnRH agonists can be used instead of hCG to provide the final maturation of the oocyte if there is a significant risk of OHSS. Under general or local anesthesia, sampling of the fully mature oocyte occurs nowadays with Intravaginal Ultrasound-guided needling of the ovaries, while the laparoscopic route is used if ovaries cannot be accessed transvaginally [10].
Fertilization of the egg occurs by insemination or ICSI beyond which they are incubated under strict laboratory conditions. Transfer of embryos traditionally occurred on day 2 or 3 following egg collection (25% implantation rate), but there is good evidence that transfer on day 5 (blastocyst stage) results in a higher implantation rates (40%) (figure 1a, b, c). The rationale for this is that physiologically embryos would be present in the uterine cavity by day 5 and implanted by day 6 to 7, which provide similar conditions to normal pregnancy. Finally a pregnancy test would be performed around 2 weeks from embryo transfer. With positive results, patients should be offered a TVS 14-21 days later to confirm if pregnancy is intrauterine and assess its viability. In case of initial low hCG, it is repeated 48 hours later to determine its rise, which if suboptimal, then ectopic pregnancy or miscarriage are possible reasons and an appropriate follow-up is required [7]
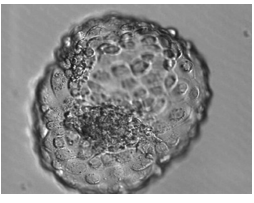
Figure1: a) shows the blastocyst stage at day 5

Figure1: b) shows the four cell stage at day 2
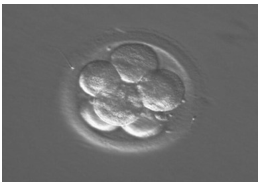
Figure1: c) shows the eight cell stage at day 3
Pictures are reproduced from “Dewhurst’s Textbook of Obstetrics and gynecology, 2017 copyrighted”
Anti-Müllerian hormone (AMH) is considered a good predictor of ovarian response in IVF. According to NICE guidelines, levels of 0.8 ng/ml or less predict a poor response to ovarian stimulation, while levels equal to or greater than 3.6 ng/ml predict a good response. Even though AMH can be measured anytime during the menstrual cycle, and does not change between cycles, its use should be combined with antral follicle count and ovarian volume, to avoid a high false positive result [11].
Embryology of implantation:
Implantation is a highly regulated process through which the embryo attaches to the surface of endometrium and penetrates the epithelium and the maternal circulation to form the placenta. It occurs in three stages: 1) apposition, 2) adhesion, 3) invasion (figure 2). Before implantation occurs, estrogen (proliferative phase) and progesterone (receptive phase) prepare the uterus and make it a favorable environment for the embryo. This occurs through proliferation of the epithelium, vascular endothelium and stroma, and removal of cell surface glycoprotein mucin 1 (MUC1), which inhibits adhesion of blastocyst. Disruptions of estrogen and progesterone action have repetitively demonstrated impacts on pregnancy rates in assisted reproduction.
 |
Figure 2 shows the different stages of implantation. Figure is reproduced from “Reviva Infertility and IVF clinic” copyrighted.
Apposition of blastocyst occurs typically in the upper and posterior wall of the uterus. Mediated by Prostaglandin E2, apposition is known to be a pro-inflammatory stage in which the vascular permeability of the endometrium is increased at the site of attachment. Dysfunctional endometrial prostraglandin synthesis has been correlated with cases of RIF [85]. The blastocyst then differentiates into an inner cell mass and a trophoectoderm. Afterwards, decidua cells form when the stromal cells surrounding the implanting blastocyst differentiate (decidualization).
These cells have a pivotal role in the physiology of implantation as well.
Adhesion of the blastocyst trophoectoderm and endometrial epithelium occurs and is mediated by cell adhesion molecules such as integrin, E-cadherins and selectins. These molecules interact with ligands produced by the extracellular matrix (ECM) of decidua. E-cadherin plays a pivotal role in the initial attachment process, lack of integrin can contribute to unexplained infertility in women and antibody-mediated inhibition of L-selectin prevents adhesion between trophoblastic tissue and endometrial epithelium.
Invasion, the last of the three steps involved in implanation, occurs when the trophoblastic cells migrate and invade into the decidua. Invasion aims to rebuild the maternal spiral arteries, replacing them with low resistance, large vessels. By the time of invasion, the trophoblastic cells have already divided into the cytotrophoblast and syncytiotrophoblasts. Disorders of invasion have been correlated to poor pregnancy outcomes as in the case of growth restriction and pre- eclampsia [12].
We searched the database PubMed using a combination of the following key words “Embryo implantation” and “Fertilization in Vitro”, “Complications”, and the initial screening results showed 2533 articles. We used the filtration tool bar in the website modifying the initial screening results according to the following “Free full text”, “Publication in last 10 years”, “Clinical Trials”, “Review articles”, “Systemic Reviews and meta-analyses”, and the search entries dramatically were shortened to 85 articles. Of these, we excluded 65 articles as they were irrelevant to our topic. The remaining 20 articles were retrieved and used in this narrative review.Literature review: Etiologies, diagnosis and treatment of unexplained RIF
Endometrial function and chronic endometritis.
The endometrial thickness is strongly related to the success of implantation, where most studies have shown that no pregnancy was possible when the pre-ovulatory endometrial thickness (ET) was < 6 mm [13]. Other studies speculated that a successful ART is usually only achieved with ET> 8 mm. Doubling this ET value increases pregnancy rates from 53% to 77%. Miwa et al. conducted a Doppler TVS study in which uterine spiral artery resistance index was significantly higher in patients with ET < 8 mm than with normal ET > 8 mm, (p-value <0.05) [14]. Women with chronic endometritis, a common an clinically silent disease, treated by antibiotics have shown improved implantation rates, clinical pregnancy rates and live birth rates after normalization of the endometrial anatomy, as reported by two studies [59][84]. Kushnir et al. discovered that among a cohort of infertile women, 45% had CE, specifically those with diagnosed RIF [53]. Even with apparently normal endometrium on clinical assessment, an altered local microbiota ecosystem, has been identified to significantly affect receptivity of the endometrium to implantation [15]. Inducing endometrial injury by dilatation and curettage (D&C) and subsequently regenerating it using hormonal therapy that produces decidualization of stroma, and release of cytokines or growth factors via an aseptic inflammatory response have no clear evidence of improved rates of implantation [16][17]. Previous trauma, protracted use of contraceptive pills and dysfunctional uterine blood flow are all implicated in the pathologically thin endometrium. Several therapeutic approaches with the purpose of increasing endometrial implantation site blood flow were suggested to treat the problem of reduced endometrial thickness as a cause of RIF. Even though therapies such as low dose aspirin, and vaginal sildenafil, high doses vaginal and oral estrogen have shown improvements in some studies, they still did not significantly enhance the reproductive outcomes [18][19].
Although not a so much literature was published in this factor, a recent study by Radzinsky, et al. 2010, found that in women with unilateral endometrioma (n=70), the ovarian reserve function as measured by AMH was significantly lower than the control group (n=50) (P< 0.005), with no statistically significant difference in the number of good quality embryos [20]. Implantation rates was significantly higher in the control group, (15.8% vs. 24.0% p<.005). Another study conducted by Brosens J, et al, assessed the role of Apo- lipoprotein A-I (Apo A-I) and found that it acts as an anti-implantation protein and seemed to be expressed in abnormally high amounts in
patients with endometriosis [21]. Supported by the data of other studies, endometriosis could be targeted as a causal factor for RIF [22][23]. Moreover, a recent systemic review shows that salpingectomy significantly rises the rates of pregnancy in women with hydrosalpinges before attempting IVF, as compared to no treatment. [24]. Defective expression of adhesion molecules such as upregulation of interleukins (IL) and high IL-1β and low interferon-γ and IL-10 is associated with RIF. High endometrial NK cells, IL-1beta, and low Interferon gamma and IL-10 were all detected in endometrium of RIF patients. No therapeutic approach has been found up to date [21][25][26].
While acquired hypercoagulability states such as anti-phospholipid syndrome (APS) have been correlated with increased rates of miscarriage, recent studies have found appreciable levels of APS antibodies in patients with RIF, but did not a reveal significant association with clinical pregnancy rates or live birth rates [27]. Thrombophilias, on the other hand, have been studied by larger trials adapting more recent investigative tools and revealed an increased association with RIF, and poor pregnancy outcomes [28]. Azem F, et al. reported higher rates of hereditary thrombophilic states such as methylene tetrahydrofolate reductase (MTHFR) deficiency, prothrombin deficiency, factor V Leiden, and ATIII deficiency in women with RIF as compared with controls (P = .012) [29]. Although one may indeed presume that the use of LMWH in such patients would be associated with lower RIF and increased success of ART as revealed by small clinical trials, an equal amount of studies on the therapeutic benefit of this drug did not reach a consensus [16][29]. Use of LMWH prophylaxis is however recommended for safety of patients undergoing IVF during controlled ovarian hyperstimulation, due to the thromboembolic risk of the hyper-estrogenic states imposed on them [1].
Associations between immune dysfunction and RIF have been established, though without solid proof, by a number of studies. A study by Elram, et al. found that couples with at least seven unsuccessful embryo transfers sharing at least three HLA loci have benefitted from 18 course of administration of nonspecific IVIG before oocyte retrieval and before detecting fetal heart rate. This study also concluded that use of IVIG therapy is safe and should potentially be considered in patients with high order RIF, HLA similarity and maternal tolerance to paternal antigens [30].Santillan et al. have studied the effects of uterine NK cells and found that they were significantly increased in patients with idiopathic RIF as compared to controls (53% RIF, 5% controls) [31]. A recent meta-analysis by Seshadri et al, however, found conflicting data regarding the association of uterine and peripheral NK cells with infertility, and live birth rates [32]. In another study by Roussev RG, et al. infusion of intralipid solution efficiently reduced NK cell activity and subsequently enhanced the implantation rate, showing promising results as a treatment in patients with RIF [33]. Not so long after that, Shreeve N, et al suggested that the use of intralipid treatment should be with caution, and the efficacy of which is yet to be proven [34]. Moreover, to enhance the pregnancy outcomes in women with RIF, a different modality of treatments, peripheral blood mononuclear cells’ (PBMCs) instillation, has been studied. A meta-analysis conduction by Yang Wu, et al. found that PBMCs use significantly improved life birth rates, clinical pregnancy and implantation rates, though its use is only restricted to research purposes [35]. In another prospect, two large studies respectively studied the association between circulating cell-derived microparticles (cMPs), and polymorphism in the Nuclear Factor Kapp B gene with RIF, and found that these cMPs were abnormally expressed in patients with RIF, reflecting in an increased risk of thrombotic events [36][37]. In any case, it is recommended that testing for immunological dysfunction be performed last in the investigations due to the difficulty in establishing a relation with RIF.
Significant chromosomal abnormalities, defined as three or more chromosomes with aneuploidy, are more likely to be found in women having RIF [38]. Though its results are mostly normal, karyotyping is important for men with severe infertility, and is recommended only in nulliparous women with RIF. Pre-implantation Genetic Testing Diagnosis (PGD) is should preferably be sought if karyotyping results are abnormal [39]. Another supportive association for the notion that chromosomal aberrations are directly related to RIF is that it was reported that the use of pre- implantation genetic screening (PGS) to select chromosomally normal embryos for transfer significantly increases implantation rates [40]. Three years later, a large RCT by Wilding M, et al. found opposing results, with PGS resulting in significantly lower rather than higher clinical pregnancy rates and live birth rates [41]. Evidence of male contribution to embryo incompatibility with implantation is present. Increased sperm DNA fragmentation was correlated with reduced
fertilization and pregnancy rates, and poor morphology of sperms was also correlated with high sperm DNA instability [42][43]. DNA fragmentation can be targeted to improve sperm quality, particularly with by use of oral anti-oxidants [44]. Alternatively, sperms with lower levels of DNA damage can be selected from the semen sample by techniques like annexin-V column and used in the fertilization process as shown by Sakkas, D, et al [45].
In other instances, the underlying cause of RIF relates to poor oocyte quality resulting in suboptimal response to ovarian hyper-stimulation. This leads to the acquisition of a high number of immature oocytes. Age-associated decline in oocyte quality results from a higher chance of acquiring chromosomal non-disjunction, and high mitochondrial DNA damage. Due to their ability to express VEGF and prostaglandins at the site of implantation, cumulus cells are also associated with improved implantation and pregnancy outcomes [46]. The role of zona pellucida (ZP) in implantation process is essential. Benkhalifa et al., 2012 reported that culturing cumulus cells along with the embryo improves implantation rates in women with RIF [47]. The process involves thinning out of the wall and rupture of ZP, which allows the blastocyst hatch out and attach to the implantation site. Even though early reports suggested that impairment of this process may result in reduced rates of implantation, and assisted hatching (AH) did show apparent improvements in the rates of successful implantation, the universal use of AH is not recommended as it is not free of complications. It is however suggested that AH use is beneficial in special populations such as those with poor prognosis, more than two unsuccessful IVF cycles, poor quality of embryo, and advanced maternal age [47][48]. Embryo culture and transfer on day 5 after egg collection has been recommended by large prospective trials, as this significantly improves implantation rates and live birth rates [50][51]. However, embryo culture until the blastocyst stage (around day 5) causes a higher rate of embryo transfer cancellation. As the use of the most highly efficient embryo transfer techniques is warranted especially in RIF patients, embryo transfer with soft non-traumatic catheters guided by US is most accepted internationally. Sallam HN, et al. As such, reviews of the previous details of embryo transfers should be sought, focusing on any history of technical issues encountered [52].
Diagnosis of RIF:
The diagnostic approach of recurrent implantation failure starts with measuring ovarian reserve function by basal FSH, AMH, and antral follicle count, karyotyping the couples with RIF and then is modified according to suspected causal factor, (e.g. uterine, paternal, immunologic, genetic or embryo related, etc). In uterine factors, the best diagnostic modality is hysteroscopy, even though hysterosalpingography (HSG) and saline sonohysterography (SHG) are less invasive, and are considered first in the approach [6]. The use of HSG in cases of RIF is warranted for detecting cases of hydrosalpinges, as HSG has a fairly good sensitivity for detecting it.
Studies have shown that treatment of hydrosalpinges by salpingectomy significantly increases successful implantation rates, pregnancy rates, and live birth rates [54][55]. A trial reported that using hysteroscopy in women with prior failed embryo transfers improves implantation rates, and clinical pregnancy, irrespective of the presence of uterine pathology [56]. However, a multicenter randomized clinical trial conducted by El-Toukhy, et al, found that in women with normal transvaginal ultrasound findings, hysteroscopy does not improve live birth rates, and further research is needed in this regard [57]. A recent meta-analysis conducted by Mao X. et al, found that hysteroscopy only improved implantation and clinical pregnancy rates, while not affecting live birth rates [58]. Furthermore, hysteroscopy with endometrial sampling is sensitive for detecting cases of chronic endometritis (CE), a known cause of RIF. In such cases, hysteroscopy could be judged to improve pregnancy rates after proper treatment of the CE by antibiotics [59][60]. The use of endometrial sampling to diagnose occult or obvious endometrial pathologies is supported for all patients with RIF [61]. Additionally, The dual function of hysteroscopy, being both diagnostic and therapeutic, made it useful in many ways, one of which is to cause endometrial scratching in the luteal phase of the cycle before IVF treatment in order to improve implantation rates [1]. Other investigations such as sperm DNA analysis is used only in clinically appropriate scenarios as mentioned elsewhere.
Two other investigations, BCL6 testing and endometrial receptivity analysis (ERA) are suggested in patients with RIF. A clinical trial in patients with infertility due to unknown cause by Almquist et al. revealed a significantly higher live birth rate following IVF (11.5% versus 58% in patients with and without elevated BCL6, respectively) [62]. Another study by Likes et al. found that using GnRH agonists or surgical treatment in patients with endometriosis-associated infertility and high BCL6 levels greatly enhances pregnancy rates. This supports the use of this marker in the algorithm of RIF management [63]. In another respect, the rationale behind using endometrial receptivity analysis in RIF patients is that the favorable window of the endometrium being receptive to implantation is present for a specific and short period (usually four to five days beginning around six days of ovulation), governed by hormonal exposure. [64] Endometrial receptivity analysis results of 238 genes were published by, Diaz-Gimeno et al. Data showed that 25% of patients with RIF had an altered window of implantation (WOI). Fouled with false positive results, abnormal ERA can also be present in 12-15% of normal control patients [65][66]. Results from a large study showed that in patients undertaking their first IVF cycle, the use of ERA shows improvement in clinical pregnancy rates but not live birth rates [67]. Thus, further studies regarding the use of ERA are needed before it can be universally adapted.
Treatment of unexplained RIF:
Although we have discussed a number the treatment methods in the above discussion, a wide range of experimental treatment modalities exists, the efficacy of most of which have not been proven to date. Even though treatment of RIF is cause-specific, there is no agreed-on protocol, and a multidisciplinary approach should always be sought. Reassurance to the couples that their issue is in the hand of skilled clinicians, and offering sufficient counselling to the couples are of the most important tasks before commencing therapy. In many of patients with RIF, a specific etiology will not be found [61]. In patients with unrecognized endometriosis as a cause of RIF, hysteroscopy could be used after prolonged pituitary downregulation, especially if grouped with the presence of disabling dysmenorrhea, abnormal BCL6 or miMRA tests [63][68]. Use of intra- cavitary hCG could be utilized as a tool to improve implantation rates and pregnancy outcomes as well. A study by Strug, et al. has shown that there is an improvement in local estrogen and progesterone responsiveness, and in other factors having a direct role in implantation, in the period following the use of hCG [75]. Other studies approve of this notion, and conclude that hCG use improves rates of clinical pregnancy and live birth. Nonetheless, they constitute a small number of trials with small populations, and more large scale clinical trials are needed in this topic [69][70]. In other trials, the use of granulocyte colony stimulating factor (GCSF) in RIF has shown promising results, in which it is used to improve endometrial thickness. In an Unblinded RCT, specifically, GCSF has shown a significantly improved implantation rate when administered 1 hour prior to embryo transfer on day 3 after egg collection [71][72]. Plasma rich in platelets has also been used for the same purpose of improving endometrial thickness through enhancing regeneration of tissues [73][74]. A large RCT, conduted by Nazari, et al. supported a positive role for this therapy, but results across other studies are inconsistent and need further research [76]. Aromatase inhibitors such as lantrozole are hypothesized to be of effective use in patients with RIF due to occult endometriosis, as aromatase expression is increased at the level of endometrium. A large cohort study by Steiner N, et al. supported this association showing increased live birth rates in RIF patients taking letrozole as compared to GnRH analogue alone [77]. Likewise, some studies greatly support the use of growth hormone (GH) therapy as it promotes receptivity of endometrium [78][79]. A study by Altmae, et al. showed increased pregnancy and live birth rates with GH treatment as compared to no treatment. Though data are encouraging as this therapy is not hazardous, more studies are needed for better characterization of the role of this GH in patients with HIF [80]. From another aspect, it is known that overreaction of the local immune system along with altered innate and humoral inflammatory responses are associated with implantation failure and pregnancy loss [81]. Steroids have, therefore, been put into practice as their anti-inflammatory, and immunosuppressive effects were speculated to enhance implantation rates in patients with RIF. However, most studies have not found a clear benefit from the use of steroids for this purpose [82]. In fact, their use has been strongly disapproved by many studies. On the other hand, Tacrolimus, an immunosuppressive agent, has shown encouraging results in improving implantation rates, due its effect on establishing a certain balance between Th1 and Th2 cells essential to the implantation process. The study by Nakagawa et al. found that women with high Th1/Th2 ration treated with Tacrolimus resulted in a 45.7% implantation rate, and 60% live birth rate, as compared to 0% success rate in both live birth and implantation in those without treatment [83].
Diagnosis and treatment of recurrent implantation failure is challenging. A multidisciplinary approach is frequently needed, especially in cases with unexplained RIF. Diagnostic investigations of this condition are widely present, some of which have not proven their efficacy until now. Although the vast majority of cases having no exact etiology, endometrial dysfunction is the most likely cause of explainable RIF, necessitating a full workup of endometrium by the different methods such as endometrial sampling, hysteroscopy and ERA. Currently, there is no established protocol for approaching patients with RIF, making diagnosis even more challenging.Proven treatment methods exist, especially for straightforward cases while unproven modalities are under research up to now.