Pediatrics and Child Health Issues
OPEN ACCESS | Volume 5 - Issue 2 - 2025
ISSN No: 2836-2802 | Journal DOI: 10.61148/2836-2802/JPCHI
Elsayed I. Salama1,2,3, Mohamed H. bahbah4, Behery E. behery1, Wallaa Mohamed4
1National Liver Institiute
4Faculty of Medicine
2Menoufyia University, EGYPT Weill Cornell Medicine College (WCMC). Qatar
3Hamad Medical Corporation (HMC), Qatar
*Corresponding author: Elsayed I. Salama, Neonatology Hamad Medical Corporation (HMC), Weill Cornell Medicine College (WCMC) Qatar.
Received: June 02, 2021
Accepted: June 14, 2021
Published: June 16, 2021
Citation: Elsayed I. Salama, Mohamed H. bahbah, Behery E. behery, Wallaa Mohamed. “Assessment of Trans-cutaneous bilirubinometry in preterm infants: Single-center experience”, J Pediatrics and Child Health Issues, 2(4); DOI: http;//doi.org/03.2021/1.1023.
Copyright: © 2021 Elsayed I. Salama. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Aim: We aimed to assess the transcutaneous bilirubin (TcB) measurements in preterm neonates versus total serum bilirubin (TSB) testing. The study carried out in our neonatal intensive care unit (NICU), on 51 babies.
Methods: According to the gestational age we classified the neonates to Group І: early preterm infants of gestational age ranged from 30-33 weeks, Group П: Late preterm infants of gestational age ranged from 34-36 weeks. According to screening time: Group Ia: preterm infants > 72 hours of age. Group IIa: preterm infants ≤ 72 hours of age. Exclusion criteria: Infants ≥ 37 weeks, infants exposed to phototherapy or exchange transfusion. TcB measurements done within 45 minutes of blood sampling for total serum bilirubin (TSB).
Results: There was a significant positive correlation between TSB and TcB readings in a preterm infant with gestational age 30-36 weeks and in preterm infants of ≤ 72 hours of age and of > 72 hours of age, also we found the best site for TcB measurement is the sternum.
Conclusions: Transcutaneous measurement of bilirubin decreases cost, pain and discomfort for the infants and their parents, and it is considered reliable method in screening and follow up of neonatal jaundice versus total serum bilirubin measurement.
Introduction:
Neonatal jaundice occurring in up to 60% of term and 80% of preterm newborns in the first week of life, and it is a very common condition worldwide [1]. Although the gold standard remains the measurement of TSB, this method, however, is invasive, painful and costly in terms of workload, time and money. Moreover, repeated blood sampling may lead to significant blood loss, which may be of particular concern in preterm infants. Non-invasive methods of bilirubin measurements have been proposed, to overcome these drawbacks [2]. Transcutaneous bilirubin (TcB) measurements readings are instant and can avoid delay of discharge and/or indicate the need for formal TSB testing [2].
Materials and Methods:
This cross-sectional study will be carried out in the neonatal intensive care unit (NICU) of Shebeen-ELkom Teaching Hospital, Egypt. According to the gestational age (GA), we classified the neonates to Group І: early preterm infants of GA ranged from (30-33) weeks, Group П: Late preterm infants of GA ranged from (34-36) weeks. According to screening time: Group I: preterm infants > 72 hours of age. Group II: preterm infants ≤ 72 hours of age. Exclusion criteria: Infants ≥ 37 weeks, infants exposed to phototherapy or exchange transfusion. All patients subjected to, in addition to full medical history and thorough full clinical examination, the following investigations were done, complete blood picture, C reactive protein, total serum bilirubin (TSB), direct and indirect bilirubin level and reticulocytes. Transcutaneous bilirubin (TcB) measurements using the Minolta Air-Shields JM-102 device (Beget Medical, Cliff Gordievsky, USA, AZ), within 45 minutes of blood sampling for TSB. Statistical analysis of the results done.
Results:
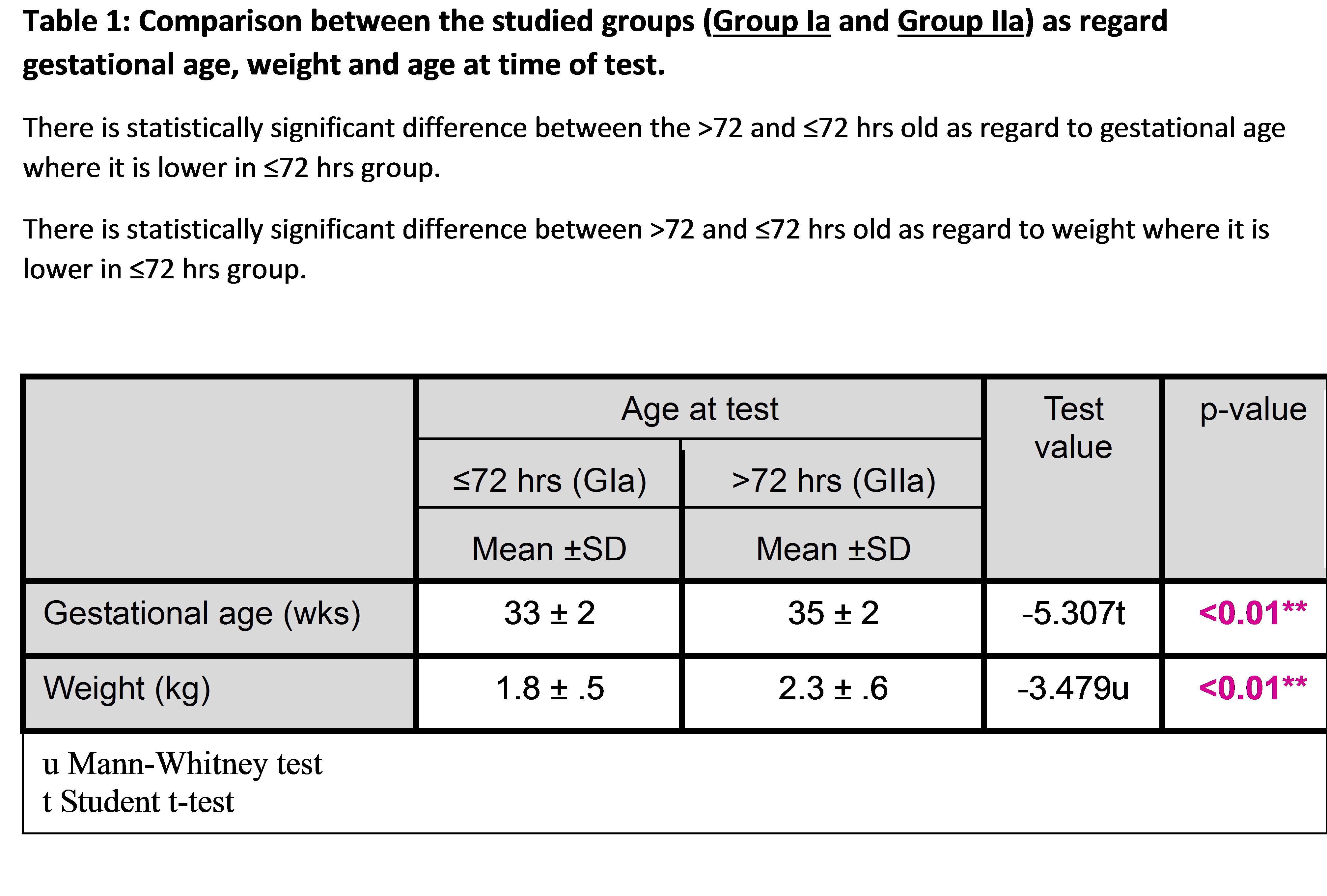
The tables (1,2,3) show that there is no statistically significant difference in sex distribution, weight for GA and mode of delivery in the early preterm in comparison with the late preterm. There is no statistically significant difference as regard sex, mode of delivery and weight for GA in the group of >72 hours in comparison with the group of ≤72 hours. However, the ≤72 hours group have a significantly higher percentage of early preterm newborns than the other group. Comparison between the studied groups as regard gestational age, weight and age of screening. There is a statistically significant difference between the group of >72 hours and the group of ≤72 hours old as regard to gestational age where it is lower in the group of ≤72 hours. There is a statistically significant difference between the group of >72 hours and the group of ≤72 hours old as regards the weight where it is lower in the group of ≤72 hours. Transcutaneous Jaundice Meter measurement show Significant positive correlations between TSB and TcB readings in a preterm infant with gestational age 30-36 weeks and in preterm infants of the group of > 72 hours of age and the group of ≤ 72 hours of age. Regarding TSB readings, it is significantly higher in late preterm compared to early preterm, where there is no significant difference between early and late preterm groups regarding TcB readings. The best site for measurement (means of three reading) in early and late preterm was sternum, particularly the late preterm group and that of ≤72 hours group, where the mean of TcB readings are better than other areas. Knee TcB reading is significantly lower than TSB level in all preterm.
|
overall accuracy % |
PPV % |
NPV % |
Specificity % |
Sensitivity % |
Tc. reading |
Patient group |
|
59.00 |
97.44 |
34.43 |
95.45 |
48.72 |
forehead |
all patients
|
|
69.00 |
92.31 |
54.10 |
91.67 |
56.25 |
knee |
|
|
93.75 |
93.94 |
92.31 |
66.67 |
98.94 |
sternum |
|
|
78.00 |
58.97 |
90.16 |
77.46 |
79.31 |
mean |
|
|
44.90 |
92.31 |
27.78 |
90.91 |
31.58 |
forehead |
GI early preterm group
|
|
61.22 |
92.31 |
50.00 |
94.74 |
40.00 |
knee |
|
|
77.55 |
46.15 |
88.89 |
82.05 |
60.00 |
sternum |
|
|
77.55 |
84.62 |
75.00 |
93.10 |
55.00 |
mean |
|
|
72.55 |
100.00 |
44.00 |
100.00 |
65.00 |
forehead |
GII late preterm group
|
|
76.47 |
92.31 |
60.00 |
88.24 |
70.59 |
knee |
|
|
78.43 |
65.38 |
92.00 |
71.88 |
89.47 |
sternum |
|
|
80.39 |
92.31 |
68.00 |
89.47 |
75.00 |
mean |
|
|
46.03 |
92.86 |
32.65 |
94.12 |
28.26 |
forehead |
GIa ≤72 hrs
|
|
60.32 |
100.00 |
48.98 |
100.00 |
35.90 |
knee |
|
|
84.13 |
57.14 |
91.84 |
88.24 |
66.67 |
sternum |
|
|
76.19 |
100.00 |
69.39 |
100.00 |
48.28 |
mean |
|
|
81.08 |
100.00 |
41.67 |
100.00 |
78.13 |
forehead |
GIIa >72 hrs
|
|
83.78 |
88.00 |
75.00 |
75.00 |
88.00 |
knee |
|
|
67.57 |
60.00 |
83.33 |
50.00 |
88.24 |
sternum |
|
|
83.78 |
84.00 |
83.33 |
71.43 |
91.30 |
mean |
Sternum TcB
readings have better results in all groups particularly late preterm group and <=72 hours group.
Table 2: Site, Sensitivity and specificity of trans-cutaneous reading of 10 mg/dl to predict Total serum bilirubin reading of 10 mg/dl in patient groups.
|
Total serum bilirubin(mg/dl) |
TcB. reading |
Patient group |
|
|
Sig. |
r |
||
|
<0.01** |
.712** |
Forehead transcutanous reading |
all patients |
|
<0.01** |
.644** |
Sternum transcutanous reading |
|
|
<0.01** |
.719** |
Knee transcutanous reading |
|
|
<0.01** |
.802** |
Mean of transcutanous readings |
|
|
<0.01** |
.523** |
Forehead transcutanous reading |
GI early preterm group |
|
<0.01** |
.507** |
Sternum transcutanous reading |
|
|
<0.01** |
.573** |
Knee transcutanous reading |
|
|
<0.01** |
.665** |
Mean of transcutanous readings |
|
|
<0.01** |
.811** |
Forehead transcutanous reading |
GII late preterm group |
|
<0.01** |
.756** |
Sternum transcutanous reading |
|
|
<0.01** |
.787** |
Knee transcutanous reading |
|
|
<0.01** |
.866** |
Mean of transcutanous readings |
|
|
<0.01** |
.554** |
Forehead transcutanous reading |
GIa ≤72 hrs |
|
<0.01** |
.545** |
Sternum transcutanous reading |
|
|
<0.01** |
.682** |
Knee transcutanous reading |
|
|
<0.01** |
.727** |
Mean of transcutanous readings |
|
|
<0.01** |
.798** |
Forehead transcutanous reading |
GIIa >72 hrs |
|
<0.01** |
.804** |
Sternum transcutanous reading |
|
|
<0.01** |
.690** |
Knee transcutanous reading |
|
|
<0.01** |
.863** |
Mean of transcutanous readings |
|
Significant positive correlations are present between TSB and TcB. readings in study groups.
Table 3: Correlations between total serum bilirubin level and trans-cutaneous readings in study group.
Discussion:
The accurate measurement of bilirubin concentrations is essential for the diagnosis of hyperbilirubinemia and for guiding the clinician regarding treatment. Trying to overcome the drawbacks of repeated sampling for TSB, non-invasive methods of bilirubin measurements have been proposed [2]. Many preferred TSB as a screening test to identify preterm infants at-risk for hyperbilirubinemia, but the clinician should consider using daily monitoring of jaundice progression, by periodic TcB testing, to minimize over-testing and overuse of phototherapy [3,4]. We aimed to assess the transcutaneous bilirubin (TcB) measurements in preterm neonates versus serum bilirubin testing.
In our work, transcutaneous jaundice meter measurement, show significant positive correlations between TSB and TcB readings in a preterm infant with gestational age 30-36 weeks (early and late preterm) and in preterm infants of > 72 hours of age and of ≤ 72 hours of age. Although, a 2013 systematic review suggested that TcB devices reported similar reliability in estimating TSB in preterm infants less than 37 weeks GA, subsequent data showed that the correlation between TcB and TSB decreases with decreasing gestational age [5,6,7]. For extremely preterm infants (<30 weeks GA), the correlation of measurements between TcB and TSB also varies depending on the body site used due to differences in tissue bilirubin binding [8]. There was a good correlation between TSB and TcB and the maximum correlation was seen in 33-37 weeks of gestation and birth weight more than 2500 g with forehead TcB measurement. Measurement may underestimate TSB, but there is a significant correlation between TcB and TSB in preterm cases even in ill neonate or who receiving phototherapy. This method can be used for the determination of bilirubin level in the preterm neonate and reduces the number of blood sampling [9]. In one study of 120 infants (mean age of 90.4 hours), there was a good correlation between TcB and TSB, they concluded that the use of TcB in the outpatient setting was a safe and reliable screen for assessing hyperbilirubinemia in infants recently discharged [10]. It was found that a moderate correlation between TcB and TSB during phototherapy with a marginal improvement in the post phototherapy phase, but further research is needed before the use of TcB devices can be recommended for these settings [11]. One study established the validity of the JM-103 meter as a screening tool for neonatal jaundice in term and late preterm infants in Mongolia, regarding versus TSB [12].
Department of Pediatrics, National Center for Global Health and Medicine, Tokyo, Japan.
Affiliations
1. Department of Pediatrics, National Center for Global Health and Medicine, Tokyo, Japan.
ORCIDs linked to this article
TcB measurements using the Draeger JM-103® device correlate significantly with TSB, regardless of term and skin colour. Transcutaneous bilirubinometry seems to be a safe and cost-effective screening method for severe hyperbilirubinemia in newborns of different terms and ethnic origins [13]. Regarding the Comparison of two transcutaneous bilirubinometers: Minolta Air-Shields Jaundice Meter JM103 and Spectrx Bilicheck (BC), TcB-JM tended to underestimate TSB levels, and TcB-BC tended to overestimate TSB levels. The sensitivity of BC was higher, but specificity was lower than JM in corresponding to different TSB levels, except at a TSB level of 15 mg/dl when both instruments yielded 100% sensitivity. The accuracy of JM in predicting TSB was higher than BC at all TSB levels. Operating the JM was simple and uncomplicated. It would be suitable for clinical use when a number of personnel perform the measurement [14]. Despite its new technology, the Bilimed® (a new transcutaneous bilirubinometer) has no advantages, and more specifically no better agreement not only in term and near-term Caucasian infants, but also in non-Caucasian and more premature infants [2].
In another side, the concern of poorer reliability of TcB with decreasing GA is further heightened because intervention thresholds become narrower with increasing immaturity. As a result, TcB screening is not widely used [3]. Thus, we do not recommend the routine use of TcB devices in extremely preterm infants (GA <28 weeks) until there are improved devices with better accuracy and precision, and a clinically validated standardized protocol for its use in preterm infants (GA <35 weeks) [15]. In another study of 87 paired measurements of TcB and TSB of term infants ≤8 days of age, mean TcB levels were greater than mean TSB (15.1 versus 13.6 mg/dL [258 versus 233 mmol/L]). In comparison with inpatient measurements, there was greater variability between TcB and TSB with outpatient measurements. In this study, the sensitivity of TcB to detect outpatient infants at risk for developing hyperbilirubinemia was 87% and the specificity was 58%. In contrast to our study, the authors concluded that further studies are needed to determine the efficacy of outpatient TcB screening [16]. However, systematic reviews have shown TcB nomogram values vary among different ethnic groups [17,18]. In outpatient setting, there are limited data regarding TcB's reliability and accuracy in identifying at-risk infants after birth hospitalization. As a result, before TcB outpatient measurements can be recommended for routine care, further studies are required to determine its efficacy and to optimize standardized protocols for its use [4]. When TcB is used clinically as a substitute for TSB, values of new instruments should always be compared with TSB measurements performed by the laboratory to ensure good correlation [19]. TcB testing may be affected by skin pigmentation. TcB overestimates TSB in infants who are dark-skinned. and might underestimate TSB in light-skinned infants [20]. At high levels of TB (>15 mg/dL [257 mmol/L]), TcB measurements underestimate TSB and need to be confirmed by standard laboratory methods. Still, TcB can replace TSB in most circumstances when TSB is <15 mg/dL (257 mmol/L) [21]. If TcB is used for screening, a confirmatory TSB should be measured in the following settings: When TcB exceeds the 75th percentile on the TB nomogram for phototherapy, If the TcB is within 3 mg/dL of the phototherapy threshold levels, At follow-up after discharge if the TcB is >12.5 mg/dL (214 mmol/L) [22]. Although genetic differences may explain the variation in TcB nomograms, differences in study designs (eg; enrollment criteria, equipment, and frequency of other risk factors [breastfeeding versus formula-feeding]) also may have contributed to the differences in the results. There are also significant variations among different instruments [23,24].
The best site for measurement (means of three reading) in early and late preterm was sternum, particularly late preterm group and that of >72 hours group, where the mean of TcB readings are better than others. Knee TcB reading is significantly lower than TSB level in all preterm. TcB measurements performed on the forehead in an infant who may have been exposed to direct sunlight may not be as reliable as an alternate unexposed site, such as the sternum [25]. Forehead TcB measurement may underestimate TSB, but there is a significant correlation between TcB and TSB in preterm cases even in the ill neonate or who receiving phototherapy [9]. Search articles by 'Hamideh Shajari' Forehead TcB correlated best with serum bilirubin levels but became less accurate at higher values. Refinements in the technology will be required before this technique, although promising, can be considered for routine clinical application in adults being evaluated for hyperbilirubinemia [26]. The differences in results may be due to personnel time for training and performing the test, and the standardization of testing, such as body location for testing [25].
Conclusion and future prospective:
Transcutaneous measurement of bilirubin concentration decreases cost, pain and discomfort for the infants and their parents, and it is considered a reliable method in screening and follow up of neonatal jaundice in early and late preterm infants. The standardization of testing is needed, and TSB should be measured in critical decisions. Further studies, advised, to compare the efficacy of different devices used.
Acknowledgments
To all nurse stuff and my colleges in national liver institute, Egypt
Conflict of Interest:
No conflict