Pediatrics and Child Health Issues
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2836-2802 | Journal DOI: 10.61148/2836-2802/JPCHI
Kulvinder Kochar Kaur1*, Gautam Allahbadia2, Mandeep Singh3
1Centre For Human Reproduction 721,G.T.B. Nagar Jalandhar-144001, Punjab, India
2Ex-Rotunda-A Centre for Human Reproduction 672,Kalpak Garden, Perry Cross Road, Near Otter’s Club, Bandra(W)-400040, Mumbai, India
3Swami Satyanand Hospital Near Nawi Kachehri, Baradri, Ladowali road, Jalandhar, Punjab.
*Corresponding Author : Kulvinder Kochar Kaur, Centre For Human Reproduction 721,G.T.B. Nagar Jalandhar-144001, Punjab, India
Received Date: March 11, 2021
Accepted Date: March 25, 2021
Published Date: March 29, 2021
Citation: Kulvinder Kaur, Gautam A, Mandeep Singh. “ An update on the Advances in the management of Congenital hypogonadotrophic hypogonadism-A Minireveview.’’. J Pediatrics and Child Health Issues, 2(1); DOI: http;//doi.org/03.2021/1.10013.
Copyright: © 2021 Kulvinder Kochar Kaur. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Earlier we have reviewed idiopathic hypogonadotropic hypogonadidsm along with special emphasis on Congenital hypogonadotrophic hypogonadism-(CHH) including the genetic mutations responsible for the same like KAL 1,kisspeptin 1KISS1, kisspeptin receptor KISSR ,prokineticin 1 (PROK 1) as well as 2 PROK2 as well as prokineticin receptor(PROKR), gonadotropin releasing hormone (Gn RH1), Gn RHR,CHD7 etc .With time advancements have been made regarding not ignoring the diagnosis till it is too late waiting for differentiation of constitutional delay in growth and puberty( CDGP) as well as CHH that wastes a lot of time for the cases of true CHH in which crucial time of minipuberty or window of appropriate therapy is already lost and severe cases may end with azoospermia besides marked psychosocial impact of failure to achieve secondary sex characteristics as well as fertility and descent of testis in severe cases .Thus here we carried out a review to update on our earlier work by doing a pubmed search for articles specifically using the MeSH terms like CHH; minipuberty; psychosocial support; Gn RH therapy; gonadotropins therapy; recombinant FSH; Cryptorchidism uni or bilateral; CDGP. We found 1435 articles out of which we selected 75 articles for this review.Thus here we emphasize on the early diagnosis s[ecially lookout for markers of CHH at birth like Cryptorchidism uni or bilateral,low FSH as well as LH at time of window of puberty around 3 mths that can be capitalized on by simulating natural physiological hormonal milieu to get spontaneous descent as well as penile growth in case of micropenis and later ensure fertility at adult age and acquisition of normal height as well as secondary sex characteristics.
1. Introduction
Earlier we have reviewed idiopathic hypogonadotropic hypogonadism ( IHH) in detail and how congenital hypogonadotropic hypogonadism (CHH),represents a rare problem that present secondary to reduced synthesis,secretion ,or action of Gn RH ,continues to be a difficult problem in paediatric endocrinology[1-7].Congenital hypogonadotrophic hypogonadism(CHH)is a rare genetic problem where reproductive disorder occurs secondary to deficiency in secretion or action of gonadotropin releasing hormone(GnRH).Basically it has been thought to be a male predominant process with a male: female gender ratio3.6:1 for which cause remains unexplained(8).The main clinical effect of CHH remain pubertal failure as well as infertility .
Though thought to be a rare disorder ,proper finding out the prevalence of CHH is not possible due to literature being scarce.On the basis of a French study of potential military service admission[9] who attended medical examination ,as well as more recently ,a retrospective study where nationwide records from Finland were collected [10], (both of which had methodologically underreporting), the prevalence of male CHH of 1 in 4415 -15,000 is right now evaluated .
The genetic deficiencies responsible for CHH mainly fall in 2 main groups , i) those leading to a neurodevelopment disorder of GnRH neuron migration usually correlated with non reproductive defects ,mainly anosmia/hyposmia from olfactory axon routing abnormally (like Kallmann syndrome[KS] and ii) that ,which lead to purely neuroendocrine dysfunction of GnRH liberation or action (normosmic CHH). Underlying this simple looking disparity lies the vast differences of genetic mutations ,with >30 genetic loci that have been thought to be involved till now ,inspite of practically half of the cases remaining unexplained.Further some genes were having implications both in normosmic CHH as well as Kallmann syndrome(KS) [11]. This complication is also seen in different ways of transmission feasible.like oligogenicity along with Mendelian forms of classical Mendelian inheritance [11.12]. Moreover CHH phenotypically remains heterogeneous.Other than different correlations with non reproductive abnormalities ,like deafness ,synkinesis(or mirror movements),renal agenesis ,digital as well as dental abnormalities as well as clefting , reproductive sequences vary from absent puberty,pubertal arrest ,to even spontaneous reversal of hypogonadism in small minority [13].Males of CHH usually come with cryptorchidism as well as /or micropenis ,that are significant property of severe fetal infancy GnRH decrease (absent minipuberty)[6]. But patients experiences point that these early presentations is rarely recognized leading to timely diagnosis as well as treatment getting started in early life.
Inspite of medical advances in genetics ,diagnostics as well as therapy health results of CHH is disappointing, with a big chunk having the long term effects of suboptimal care[14].Here we try to review the factors leading to poor results in CHH, as well as the strategies which can make the quality of life (QOL) as well as fertility potential, with special concentration on utilizing the window of minipuberty for early diagnosis as well as intervening.
2. Delay in Diagnosis as well as Therapy
Delayed puberty is the main method of presentation in CHH men. About 2/3rd of CHH men-adolescents do not display any evidence of spontaneous puberty at >17yrs of life (testis volume <4ml), with the rest showing arrest of puberty [15].
It is unfortunate that biochemically CHH can’t be separated from constitutional delay in growth and puberty (CDGP) biochemically, with the latter representing 65% of Delayed puberty in young teenage boys [16], but decreased markedly with advance in age at presentation. In both low sex steroids correlated with low or inappropriately normal follicle stimulating hormone (FSH) as well as luteinizing hormone ( LH) amounts. As far as height is concerned ,the baseline height SD scores as well as growth velocities don’t seem to be significantly variable among CDGP as well as CHH adolescents, varying from functional HH where there are a chances of lower height SD scores as well as decreased growth velocity (<3cm/yr [16]. But progressive decrease in height SDs has been seen in few CDGP boys in the prepubertal years with final height achieved remaining short of their genetic potential [17]. Conversely preservation of height relative to parental height in CHH has been pointed [18]. Thus proper history of growth can add significant information.
Despite different stimulation tests (like GnRH stimulation test as well as HCG test) as well as inhibin B(IB) amounts (marker of sertoli cell function)have been posited, although still no agreement on the ‘’gold standard’’ test to separate them reliably 19].Thus diagnosis of CHH continues to be a tough job,with clinicians usually move towards the expectant management, giving adolescents adequate time for the ones with CDGP to go through spontaneous pubertal initiation, to identify those having CHH[20].In big single centre retrospective series the combining of testicular volume as well as basal IB amount ,both can be got easily without utilization of complex dynamic test showed aid in separating CDGP as well as CHH and hence could be useful for broader use[reviewed in [2,3].
But constant misuse of “wait as well as watch & reassure’’ guide (meant for subjects of undifferentiated pubertal delay) from those with red flag markers of CHH who need ,rather than get assumed hypogonadal till proven otherwise and get sex hormone replacement from mean age of pubertal onset in that population i.e 12yrs in boys [13,21], while therapy should not be unnecessarily held back in those without CHH features but have reached 14yrs age [22].
Hence the CHH diagnosis as well as initiation of clinically useful therapy gets unnecessarily delayed till late adolescence or early childhood .Inspite of various surveying methods across various European countries ,data on the mean age of CHH who have been Markedly consistent ,with Dwyer et al (web utilized, pan European; n=101) observed these to be 18±6 as well as 19±5 yrs respectively [22],while Raivio et al (utilizing national Finnish cohort study)observing the median age of initiating therapy to be 18.3yr(range 11-34yrs)[23], as well as Quinton (case noted based survey ;n=200) observing it to be 18.9±9yrs [24].
A big mistake, commonly done was not laying significance to recognizing cryptorchidsm,where about 2/3rd had bilateral undescended testis [15,25-27] .Conversely cryptorchidsm is rarely seen in CDGP.Just 2% of CDGP boys had a history of cryptorchidsm as compared to 36% in CHH,in a large series of boys examined at a specialized centre referred from primary care for delayed puberty [27].A family history of cryptorchidsm, micropenis, Infertility as well as /or non reproductive presentations like anosmia, deafness ,synkinesis (or mirror movements), renal agenesis ,digital as well as dental abnormalities as well as clefting could aid in valuables signals if present concomitantly for early diagnosis of CHH, although this is missing in most of cases ,partially due to different penetrance of disease as well as phenotypic expression ,along with oligogenic inheritance with unaffected parents [11,rev in 3,4].
Similarly the importance of bilateral undescended testis in neonates as a probable pointer of CHH is classically not picked up.Persistence of bilateral cryptorchidsm is observed in a quarter of CHH infants in contrast to <0.7% in a the general population .In a big single centre retrospective series,only a third of CHH males with history of bilateral orchidopexy in childhood were referred to paediatric endocrinology for further examination ,with major of cases again presenting later in life with absence of puberty [25]. Present clinical guidelines have mainly concentrated on trying to analyze for congenital adrenal hyperplasia (CAH) as well as disorders of sexual development( DSD) and avoided giving enough attention ,essential for the investigations essential for cryptorchid boys for trying to exclude CHH [28] causing a lost opportunity to pick it up early.
Hence due to late diagnosis as well as delay in therapy, the proper standard of care is not given to patients with CHH,causing a significant psychosocial as well as reproductive complications.
3.Health meets not met
3.1Psychological Health
Psychological morbidities as well as antidepressant utilization are too much enhanced among men with CHH[23]An internation study with North as well as South America ,European as well as Australian participants ,practically 2/3rd were afflicted by depression, with most of them showing moderate to severe symptoms[30].A significant result secondary to chronic affective disorders is poor compliance to long term hormone replacement. What is worrying is than >a third of the survey reporters documented long gaps (>1yr) in therapy that could potentially exaggerate affective symptoms in return as a vicious cycle .
The Psychological effect of disease is marked .An important amount of patients have anxiety as well as low self esteem ,causing an inability to develop close relationships as well as social isolation .Thus in the above study cohort[30],about 50% of the men were not in a stable relationship during the survey , as well as many never had a sexual partner.
What was positive though is most of the men evaluated had got tertiary level education as well as had a gainful job showing adequate socioeconomic situation ,though this did not overcome the psychological effects secondary to the disorder. Yet men having CHH that had adopted or had biological children had <chances of depressive symptoms, pointing to the positive influence of family companions , as well as the vicious cycle broken.
The main reason that mental health as well as poor QOL is delay in diagnosing as well as not delivering age proper secondary sex characteristics in many of these CHH men.Most patients start getting therapy that is efficacious only in late adolescence (usually median age 18-19 yrs) classically following a long frustrating diagnostic journey (shared by females too though incidence lower).Thus they are at a risk of forming psychosocial disorders correlated with pubertal delay , as well as low self esteem ,social withdrawl,poor school performance as well as high risk of substance abuse disorder [22,31,32]. Moreover ,improperly treated cryptorchidsm as well as /or micropenis can cause long standing bad effect on their sexuality [23].
3.2Fertility Potential Decreased
In case of young males having CHH, gonadotrophic therapy gets a markedly > positive influence as compared to testosterone(T) replacement on health –associated QOL [33]. Although both treatments are efficacious in getting the physical as well as general health better ,those receiving gonadotrophins do better in psychosocial domains that are emotional as well as mental health ,especially if sperms are isolated within the ejaculate .This strongly points that patients psyche is markedly influenced by their anticipated chances of getting paternal hood.
In cases of CHH patients infertility is secondary to spermatogenic failure ,that can be potentially treated with either GnRH or gonadotrophins therapy .Problem is classic spermatogenic therapy –human chorionic gonadotrophins (HCG) monotherapy or combined gonadotrophins therapy (HCG as well as FSH)is much <efficacious in men with severe CHH(testes<4ml) especially those having history of bilateral cryptorchidism, rather than in men having HH of postpubertal origin like due to acquired pituitary disease[34].However in centres having experience in looking after CHH patients, till 3 quarters can acquire spermatogenesis during hormonal induction therapy [13,35], as well as pregnancy rates can get be further escalated with artificial reproductive technology(ART) [13,36].
Patients presenting with rare medical problems ,by definition when a prevalence of <5 in 10,000 in the population ,usually have it tough due to no knowledge of the care givers[37,38].Need for specialized centers having expertise in diagnosis as well as interdisciplinary therapy of rare diseases are essential in giving care to these patients [39]. On the same hand , patients with CHH need to get tertiary level care for preventing gaps in therapy , as well as benefit from latest advancements as well as technologies in the research field .Early diagnosis would give the chance for patients to get proper as well as consistent care as well as support in specialized centers without waste of time. Therapy can further be tailored as per the requirements as well as goals in various stages of life [13,40]. However in real life setup as per a survey ,it seems that only few of CHH men looking for fertility management to attain the wanted results on fertility-stimulating therapies[14]. It is not clear how many of these men were treated at specialized centres ,having essential expertise ,but knowing that only half of the whole study cohort are followed by specialized centres, access to such places is going to be restricted.
3.4. Escalated risk of Low Bone Mineral Density
Chronic sex steroid deficiency is a main risk factor for osteoporosis as well as fragility fractures which involves both sexes. Since CHH men have early onset of testosterone(T) deficiency ,a delay as well as /or absence of enough androgen replacement would aid in poor bone mass formation as well as acceleration of bone loss [41]. Patients who get T at older age seem to acquire <bone mineral as compared to younger age ,that further corroborates the significance of timely therapy [42], Even for those who get diagnosed as well as started on therapy only later in life ,encouraging BMD enhancement ,especially at trabecular rich lumbar spine [43].
4.MiniPuberty –Key Time of genitalia Formation and the Window of early Diagnosis
High chance of cryptorchidsm as well as microphallus is seen in CHH men due to absence of MiniPuberty,that is a key time in the ontogenesis of the male repro tract when activation of the GnRH axis in the early mths postnatally .At this formation phase ,serum T, as well as gonadotrophins amounts increase rapidly as well as peak at the age 3,mths –markedly correlating with adult male amounts –prior to mid childhood quiescence by about 6 mths of age [7,44,].
This marked hormonal action is essential for finishing the total event of inguino scrotal testicular descent as well as anchoring in the scrotum as well as penile growth ,that had started earlier at the time of 3rd trimester .Particularly ,LH –stimulated liberation of T as well as insulin like 3 peptide(INSL3) peptide via leydig cells are the crucial factors involved in influencing these alterations [45].A concomitant escalation of FSH stimulated IB as well as antimullerian hormone( AMH) liberation ,pointing to active proliferation of sertoli as well as germ cells as well as seminiferous tubule development are crucial determinants of future fertility potential and cause 90%of testicular volume development [46].Inspite of rapid gonadotrophin action as well as T liberation at this proliferative phase germ cells maturation as well as spermatogenesis do not take place ,since androgen receptors are not expressed on Sertoli cells till 5 yrs of age[47].
After MiniPuberty,the Hypothalamo-Pituitary-Gonadal (H-P-G) Axis reverts back into silence for the rest of childhood .Serum T,LH, as well as FSH amounts decrease to low levels till reactivation of gonadal axis in early adolescence ,pointing to the initiation of puberty as well as marked by testicular enlargement (≥4ml)followed as well as by penile as well as pubic hair growth.Here sertoli cell maturation takes place,as seen by an escalation of IB amount as well as decrease in AMH amounts ,and spermatogenesis gets attained by the coordinated effects of FSH as well as intertesticular T[48] Hence pulsatile GnRH liberation in the neonatal period seems to be of significant for the formation of male genitalia ,with a long reaching effect on male reproductive phenotype as well as fertility potential later in adult life .
Another significant clinical importance of MiniPuberty is that it gives a window of chance to promote the finding of children with Congenital GnRH deficiency ,who would show abnormally low T,LH, as well as FSH amounts on measurement,thus giving a benefit of a definitive prepubertal diagnosis as well as signposting them to preplanned pubertal induction with sex hormones at the median age of pubertal onset instead of expectant treatment followed earlier.
5.Avoidance of Delay in Pubertal Induction by Early Diagnosis
Thus Early life Diagnosis of CHH aids in structuring the long time monitoring as well as therapy plans along with seeing to it that counselling as well as psychological support to patients as well as family are given.Once patients reach early adolescence,age –related Pubertal Induction therapy will see to it that secondary sex characteristics form as it does in peers[15],thus preventing the delay which has been usually found by most CHH men.Thus uncertainties gets to the least along with removing the fear as well as anxiety.
5.2Advantage of Early Diagnosis in Escalating the Chance of Fertility
Early Diagnosis of boys presenting with CHH could probably give a chance to maximize fertility potential. Though longterm GnRH therapy or gonadotropins combinations are efficacious for most CHH men in inducing spermatogenesis, sperm results usually remain suboptimal[35,49,50].Further ,about a third with severe CHH continue to be azoospermic despite prolonged gonadotropins combinations as well as monotherapy with HCG has continued to be not efficacious to our disappointment.
Clinical Features (that corroborate with longtime severe GnRH deficiency minipuberty)which forecast poor treatment response;like total absence of puberty on presentation,ii) cryptorchidism(particularly if bilateral);low serum IB amount(pointing depleted sertoli cells) as well as prepubertal testicular volume(pointing depleted germ as well as sertoli cells as well as seminiferous tubules ) [35,49].Conversely CHH men presenting with partial GnRH deficiency (testicular volume ≥4ml)give a much better response to gonadotropins combinations, with about 80% attaining sperms in the ejaculate [51].
Hence men presenting with complete CHH ,need the maximum utilization of germ as well as sertoli cells proliferation as well as growth of seminiferous tubules by delivery of recombinant follicle stimulating hormone before the HCG gets introduced to avoid premature maturation of depleted pool of sertoli cells under the action of intratesticular T.
FSH- monotherapy in children as well as adolescents with HH of prepubertal –onset showed its effectiveness in aiding in testicular growth as well as circulating IB amounts [52]. Especially there was a good spermatogenesis response in a subgroup of adolescents having CHH where FSH priming was done prior to the combining with HCG caused a success in inducing spermatogenesis in ¾ patients [52].
Potential advantage of unopposed FSH- therapy was evaluated further in a randomized ,open label trial of 13 treatment naïve adult CHH men possessing prepubertal testis(<4ml)[53].7 men were randomized to recombinant FSH-pretreatment x4mths prior to giving a 24 mth GnRH- therapy protocol.At the time of FSH-only phase , testicular volume doubled as well as circulating IB amounts escalated to adult amounts, as well as all patients in this arm formed sperms in ejaculate on GnRH- therapy as compared to 4/6 men in the 24mths GnRH- only arm.Further trends towards > testicular volume, as well as >sperm counts along with shorter time to the 1st look of sperms in the ejaculate in the FSH- therapy pre treatment group was observed.
Hence the advantage of FSH-priming as well as potential harmful action of premature HCG would be significant to tell the clinicians on the selection of treatment would be markedly aided in timely diagnosing.
5.3 Potential of neonatal gonadotropins therapy for further enhancing outcomes
Getting insight into the key role of Mini –Puberty in the formation of external genitalia as well as sertoli cells proliferation as well as future effect on future fertility the possibility as well as advantage of bringing about the physiological hormonal milieu in male CHH infants has been evaluated.
The 1st report that was published was that a boy presenting with CHH with micropenis who was administered short term recombinant LH as well as FSH from the age of 7.9-13.7mths, the penile length enhanced successfully by 50% as well as the testicular volume almost tripled by the end of treatment [54]. Another publication of 2 male infants ,one case each of Combined pituitary hormone deficiency (CPHD) as well as CHH,6mths of gonadotropins combinations therapy was delivered via a subcutaneous pump infusion started at age 8 as well as 20wks respectively ,caused a 4 times escalation of penile length as well as testicular volume [55]. Even later ,3-6mths of continuous subcutaneous infusion of recombinant human gonadotropins in 5 male infants [4CHH ,1CPHD] gave various fold escalation of IB amounts , testicular volume as well as T liberation [56[.
Other than these good effects with of gonadotropins combinations therapy at infancy ,it could further aid in managing undescended testis . Cryptorchidism is there in about 50% of boys with severe CHH[25],being an independent anticipating factor for infertility .If orchidopexy is delayed it correlates with marked decrease in germ cells [57], and usual recommendations are there that surgery be done by 1 yr of life[58].Nevertheless ,small testis causes difficulty in surgical manipulation as well as would lead to extra risk of testicular damage as well as tissue loss [59,60].By delivering a period of presurgical gonadotropins, aids in escalation of testicular volume which helps in the procedure.
Actually there is data coming that spontaneous descent might get successfully stimulated by gonadotropins therapy in infants having central hypogonadism,thus avoid the requirement of surgery .In 8 infants having maldescended testis secondary to hypogonadototropic hypogonadism[[5CHH ,3CPHD] ,infusion of gonadotropins stimulated full testicular descent in 6 boys with partial descent in 2 boys ,so that only 1 required orchidopexy almost a yr later due to reascent of the testis[61].In another combination of recombinant LH as well as FSH in the 1st 6mths of life successfully stimulated spontaneous descent of the testis in 2 to 4 bilateral Cryptorchid CHH /CPHD boys [56].
Thus short term neonal gonadotropins therapy in confirmed cases of hypogonadototropic hypogonadism seems to be efficacious in replicating the actions of mini puberty,by correction of ,micropenis ,facilitation of testicular growth in view of sertoli cells expansion as well as stimulating spontaneous testicular descent of malpositioned testis.Significant , treatment was well tolerated in all cases documented.Though definitive proof is still absent ,the chances of early hormonal replacement in CHH boys in escalating sexual as well as reproductive function in adult life is worth serious evaluation,and give input for larger clinical trials .
This was exemplified by the study of Papadimitriou etal.,in which Neonates or infants ,all having bilateral cryptorchidism in intraabdominal/inguinal place as well as micropenis with no neonatal male minipuberty,got daily subcutaneous injections of Pergoviris(recombinant LH/FSH 75/150 IU for 3mths as part of the REMAP (REplacement of MAle mini Puberty) study where 10yr follow up was attempted .By the end of therapy ,median LH/FSH ,both undetectable prior to therapy ,went upto high normal levels of 4.45 IU/L as well as supranormal levels 83 IU/L,respectively ,median inhibin –B as well as antimullerian hormone(AMH) levels enhanced from below normal (27.8and 1.54ng/mL, respectively) to normal values (365 as well as 150ng/mL, respectively),median testosterone escalated from just detected (0.02ng/Ml) to normal values(3.3ng.mL).Stretched penile length enhanced from a median of 2 to 3.8cm .During treatment all testes descended to the scrotal position (by the end of 1st mth in 3 cases ,the 2nd mth in 4 patients and the 3rd in 3 patients )measuring 1.5ml and ,looking normal sonographically .Extra therapy with testosterone enanthate was administered to these infants.In 2 infants ,one of 2 testes regressed in the low inguinal area;both infants got successful treatment surgically .Following 1 to 10yrs of follow up ,all testes are still in scrotal position ,having slightly regressed in size .Hence the proposed regimen simulates male minipuberty and treats successfully infants presenting with micropenis as well as cryptorchidism along with restoration of sertoli as well as leydig cell function as per Papadimitriou[62].Hence from this it is quiet clear that early identification of CHH as well as isolated sertoli cell dysfunction needs to be identified in prepubertal as well as transition age .
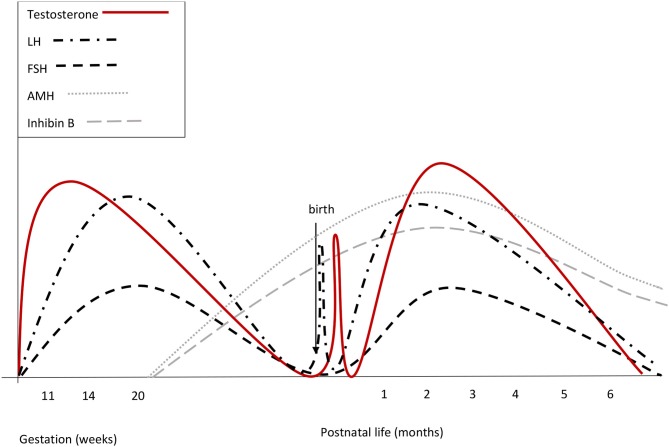
Legend for Figure 1
Courtesy ref no-74-Summary of reproductive hormone changes during early life in healthy boys. Fetal gonadotrophins surge at mid-gestation, then decline and are low or undetectable in cord blood, owing to the inhibitory effect of placental estrogens. Immediately after birth, LH transiently increases by around 10-fold, followed by a testosterone peak, which lasts 12–24 h. A few days after birth, gonadotropins surge again. LH peaks between the 2nd and the 10th week of life and then gradually decreases, reaching the low prepubertal values by 6 months of age. FSH drops to the prepubertal range within the 4th month of life. Both at mid-gestation and during minipuberty, LH predominates over FSH.
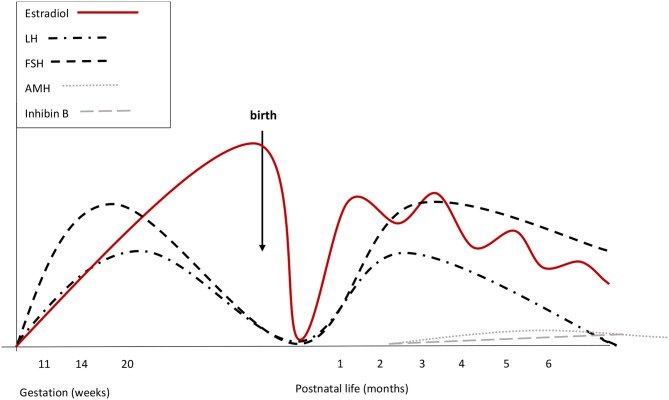 Legend for Figure 2
Legend for Figure 2
Courtesy ref no-74-Summary of reproductive hormone changes during early life in healthy girls. Fetal gonadotrophins peak at mid-gestation. Overall gonadotropin levels are higher than in males. During fetal life placental and fetal estrogens overlap. Cord blood LH and FSH are low or undetectable, due to the inhibitory feed-back induced by placental estrogens. There are no peaks of LH or estrogens immediately after birth. Starting from the 2nd week, gonadotropins increase and stimulate estradiol secretion that remains high (although fluctuating) until at least the 6th month. LH declines at the same time as in boys, while FSH remains stably high up to 3 or 4 years of age. Both at mid-gestation and during minipuberty, FSH predominates over LH.
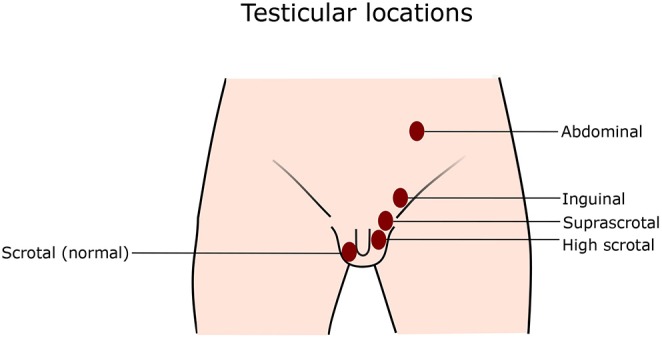
5.4 Proposed Method for Enhancing Finding the male infants with severe CHH
This phase of minipuberty gives an extraordinary useful window to confirm or deny the diagnosis of congenital hypogonadism with a simple biochemical analysis without the requirement of complicated dynamic examination.In a cohort of CPHD male infants (mainly presenting with hypoglycaemia),presence of low LH as well as FSH and T amounts reliably picked up concomitant hypogonadototropic hypogonadism in 14/15 infants with genitalia anomalies ,while all other boys with normal genitalia showed intact P-G axis function[63].This is in marked contrast with the method of differential diagnosis of CHH from CDGP in adolescence.But unlike CPHD,neonates with CHH without Cryptorchidism especially if bilateral or correlated with micropenis ,so that proper examination can be done[27,58].Actually the presence of red flag markers need more tests for ruling out such possibility.
The other problem is trying to evaluate the < obvious gonadotropin well as T results in the male infants .Of significantly ,normative values of various significant reproductive hormone reports during minipuberty –that are LH ,FSH and T(both via radioimmunoassay(RIA) as well as tandem spectrometry ),AMH well as IBamount have been calculated via a large cohort of healthy Danish infants where result amounts above the cutoff levels extensions formed lower than adult ranges –could be taken as the intact HPT axis function ,while if equivocal biochemical outcomes which lay below the cutoff levels might require testing again.Moreover expanding the screening panel well as include AMH well as IBamount could improve the confidence of diagnostic ability.Critically this study also showed that the different sex hormone peak just prior to 3.5mth of age ,pointing that diagnostic acumen would be best if analysis is done at this time.
5.5 Potenial screening indicator-Bilateral Cryptorchidism
Although routine screening for minipuberty is not practical ,a targeted approach examining infants when suspicious signs of CHH,would be cost effective.Of the Clinical Features -correlated Bilateral Cryptorchidism is especially significantly in view of its >prevalence with severe GnRH- deficiency which has both prognostic as well as therapeutic significance[15,16,26].
Though Cryptorchidism is a common congenital urogenital anomaly in newborns ,many would undergo spontaneous descent without hypogonadism /other organic problems.Results from earlier studies demonstrated that the prevalence of Bilateral Cryptorchidism reduces markedly from 1.66 to 4.54% at time of birth to 0.09-0.66% by 3-12 mths of age[64-66].Additionally spontaneous descent is not likely to occur beyond 3mths postnatal [67],that coincides with the anticipated minipuberty,that makes it an ideal time for assessing reproductive hormones in boys having continuing Bilateral Cryptorchidism.Further although non-CHH Cryptorchidism might also show hormones anomalies,they tend to have > FSH ,similar T as well as little < IBamount as compared to healthy infants ,that are separate from the biochemical pattern anticipated in CHH male infants[68].
Hence evaluating male infants[ with Bilateral Cryptorchidism with/without micropenis at 3 mths of age for absent minipuberty could aid in an appropriate approach for aiding in early CHH diagnosis . Utilizing British birth data as an e.g of mathematic evaluation
i)with an average male live birth rate(LBR) of 390,070/annum in Britain[69]-among 26 of 88 boys born every yr could be afflicted by CHH(on the basis of anticipated prevalence of 1 in 4415-15,000).
ii)Conversely , Bilateral Cryptorchidism could involve among 6475-17 ,702(54%)of all boys at birth ,irrespective of the presence of CHH.Of these among 4 as well as 30 infants could possess underlying CHH(as Bilateral Cryptorchidism involves 13,9-34.5% of CHH population),hence representing 0.02-0,46% of all Bilateral Cryptorchidism boys at birth.
iii)by 3mths of age following spontaneous testicular descent anticipated in most of non –CHH infants ,the total number of infants with Bilateral Cryptorchidism would be anticipated to reduce considerably to among 351 as well as 2,574(0.09-0.66%).
iv)Since spontaneous testicular descent is not anticipated in CHH involved infants,they now represent the >%age of all potentially Bilateral Cryptorchidism boys ;0.16-8.55%(as compared to 0.02-0,46% at birth).Thus screening at 3mths of age seems to be the most cost effective by preventing tests for maximum number of infants without persistent Bilateral Cryptorchidism
On the basis of this posit ,probably 1 in 11-12 male infants with persistent Bilateral Cryptorchidism at 3mths of age could have underlying CHH.Significantly this calculation corraborates the observations from a historical surgical patient series that had been treated with orchidopexy.Here in 98 patients examined for probable underlying endocrine etiology for Cryptorchidism,2 were seen to have CHH in adult life as well as both of them had Bilateral undescended testis [70],that represented 6.1%(2/33)of all individuals with Bilateral Cryptorchidism in the series.
Yet is needs emphasis that no literature is there pointing that CHH screening in infants has been systematically evaluated by any research group,hence remains a working concept which is in early exploratory time.Future long multicentric research is essential for any screening method that tries to get early diagnosis of this rare disorder as well as effectiveness as well as safety of hormonal therapy in CHH infants .Protocols need to be developed with adult as well as paediatric endocrinologists to make sure that these children are put in a structured follow up as well as transitional programme ,hence ensuring that no body gets a victim of the breakthrough of the health system.
6.A Little about Pre- pubertal Acquired Hypogonadotropic hypogonadism
Pubertal failure in male adolescents with known history of acquired hypopituitarism usually prevents the diagnostic testing for CHH,thus allowing Pubertal induction therapy to get planned around 12yrs of age ,with gradual escalation of T dose prior to reaching adult replacement dose in approximately 3yrs [21].Significantly in view of preserved minipuberty , normal sertoli cell proliferation is anticipated in early childhood ,testicular maldescent is most propably not going to take place[52].Hence these individuals usually have more optimism regarding fertility,with higher spermatogenic response to gonadotrophin therapy[71],until testicular tissue has been affected by previous gonadotoxic treatment.To allow a timely identification of isolated tubulopathy and sertoli cell dysfunction, the investigation should start in the prepubertal age and the transition phase[72]. The latter is the moment of transition from the pediatrician to the family doctor and hence one can avoid development of male infertility[reviewed in ref [73].
7. Conclusions
Early finding of CHH via finding the absence minipuberty can potentially manipulate the patients experience via aiding in timely treatment at various phases of life.This can be achieved in a male utilizing a systematic approach by isolation of male infants with markers of CHH especially Bilateral Cryptorchidism,so that biochemical investigations can be done in the narrow diagnostic window called the minipuberty. Bizzari and Cappa showed the difference in hormonal changes during minipuberty in male and female infants with in utero changes in their hormones [74].
Neonatal gonadotropins therapy seems to be of advantage in rectifying micropenis as well as bilateral cryptorchidism.In adolescents age proper pubertal induction is the basic target ,and a small course of FSH monotherapy needs to be tried to maximize the fertility potential.Close cooperation among paediatric care providers as well as adult endocrinologists would make sure that these patients have a smooth transition to adulthood ,at the time aim of therapy should shift towards fertility induction as well as longterm androgen replacement therapy .Equally significant are the psychological aupport a as well as genetic counselling needs to be given along the way for empowering the patients ,for coping with the situation.Rarely once kisspeptins are in use smoothly they can be tried in those with Kisspeptin or its receptor deficiency [6] .