Neurosurgery and Neurology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2025
ISSN No: 2836-2829 | Journal DOI: 10.61148/2836-2829/NNR
Raef F.A. Hafez 1, Magad S. Morgan2, Esam Abed El Kawy3, Osama M. Fahmy4, Wael K. Zakaria5, and Hamdy T. Hassan6
1Prof. of Neurosurgery and Gamma knife Radiosurgery-International Medical Center-IMC, Cairo- Egypt.
2Consultant Neurosurgery. International medical center-IMC, Cairo- Egypt.
3Consultant Neurosurgery. El Galla Military Hospital. An associate consultant -IMC, Cairo- Egypt.
4Prof of Neurosurgery. International medical center (IMC), Cairo- Egypt.
5Assistant Prof. of Neurosurgery Mansoura University Hospital. An associate consultant -IMC, Cairo- Egypt.
6Consultant Neurosurgery –International Medical Center-IMC, Cairo- Egypt.
*Corresponding author: Raef F.A. Hafez, Prof. of Neurosurgery and Gamma knife Radiosurgery-International Medical Center-IMC, Cairo- Egypt.
Received date: February 02, 2023
Accepted date: March 24, 2023
published date: May 23, 2023
Citation: Raef F.A. Hafez, Magad S. Morgan, Esam Abed El Kawy, Osama M. Fahmy, Wael K. Zakaria, (2023) “Gamma Knife Radiosurgery an Effective Safe Option for Glomus Jagulare Tumors Control: A Single Institution Long-Term Experience and Review of The Literature”. J Neurosurgery and Neurology Research, 5(1). DOI: http;//doi.org/02.2023/1.1047.
Copyright: © 2023 Raef F.A. Hafez. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objective; Glomus jugulare tumors (GJTs) are benign, slowly growing tumors, highly vascular, with the potential to infiltrate neurovascular structures. Surgical treatment is usually associated with high morbidity and even death. Gamma Knife radiosurgery (GKRS) has been established as an effective treatment option. This retrospective study aims to report and confirm GKRS.'s long-term effectiveness and safety for GJT patients. Methods; A total of 65 patients with GJTs were treated with GKRS, at the authors' center from 2005 to 2020, with a mean follow-up period of 87.7 months. The mean treated GJT volume was 5.4cc with a median prescription dose of 15Gy and a median maximum dose of 42.9Gy. Results; Most patients were females (77%), and the median age at presentation was 48 years. The overall tumor growth control was 93.8% (61 patients) 39% of them achieved tumor size reduction. The overall clinical control was 90.8% (59 patients), and 40.7% achieved clinical improvement. The Actuarial tumor rate free of progression was 100% at 3 years, 91.5% at 5 years, and 86% at 10 years of follow-up. Conclusions; GKRS for GJTs typically results in high long-term tumor control and lower neurological morbidity than those associated with microsurgical resection, therefore should be consider as a dependable effective treatment option.
Background
Glomus jugulare tumors (GJTs) are rare benign skull base tumors that arise from paraganglia adventitia on the superior surface of the jugular bulb within the jugular foramen. They typically exhibit indolent growth within the temporal bone with the potential to infiltrate the facial and lower cranial nerves (CNs), petrous bone, carotid canal and artery, and posterior fossa. GJTs represent 0.03% of all neoplasms and 0.6% of all head and neck tumors; they occur predominantly in women in a ratio of 1:1,000,000 in the fifth and sixth decade of life. [1, 2, 3, 4. 5 ]. GJTs may extend intra-cranially, compressing the brain stem, and extra-cranially into the cervical region. [1,3, 6] Early symptoms may be as subtle as pulsatile tinnitus or conductive hearing loss. With progressive tumor growth, dysphagia, dysphonia, and tongue weakness may develop as manifestations of lower CNs involvement. Additionally, patients may develop headaches, ataxia, or vomiting from elevated intracranial pressure from venous sinus thrombosis or, rarely, obstructive hydrocephalus. Ataxia and brainstem symptoms infrequently develop with larger tumors with intracranial extension [1, 5, 7, 8, 9, 10 ]. As many as 10% of GJTs may be familial, inherited in an autosomal dominant pattern with paternal genomic imprinting. [1, 11, 12, 13 ]
Fractionated external beam radiotherapy, or Radiosurgery. Traditionally, managing these tumors involved microsurgical resection that may proceed by preoperative embolization. Various procedures may result in planned staging or be used with a salvage treatment after recurrence or progression [7, 11]. All GJTs are highly vascular and develop within proximity to the pars nervosa of the jugular foramen, rendering gross-total resection (GTR) challenging with a relatively high risk of lower cranial nerve injury. [1, 3] Thus, it is not surprising that resection entails a great deal of morbidity and often leaves behind large residual tumors and even may cause mortality [1, 4, 7, 14, 15, 16 ].
Gamma knife surgery (GKRS) has been used successfully to treat GJTs and is considered a less invasive procedure that provides a better chance of cranial nerve protection and tumor control. GKRS allows the delivery of a single, biologically high-dose radiation treatment with extreme conformity (sharp dose gradient at the tumor edge). [1, 3, 6, 7, 17, 18 ] Several published series of GJTs treated with GKRS have reported excellent tumor control outcomes for both primary [1, 3, 8, 10, 15, 20, 21, 22] and recurrent tumors with preservation of lower CNs function. [8, 10, 12, 19, 20, 23, 24, 25 ]
Materials and Methods
Objective: This retrospective cohort study aims to review, analyze and report the effectiveness and safety of GKRS in treating GJTs patients treated in our center through describing a single-center long-term experience of more than 15 years.
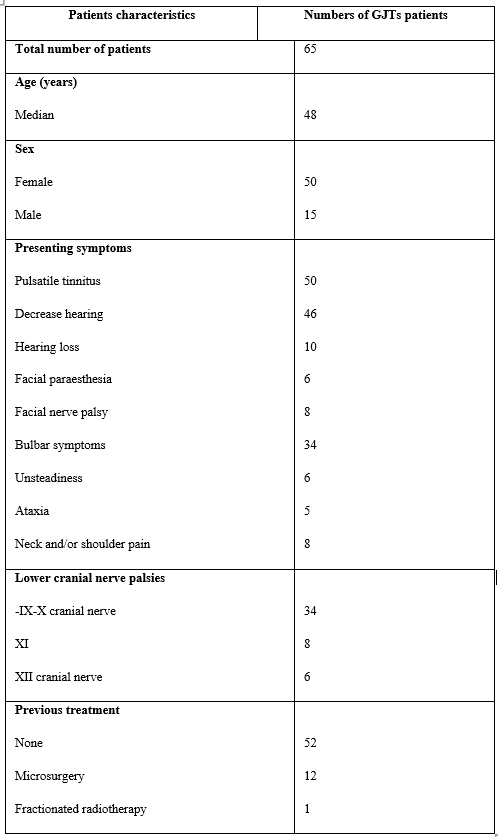
Patient population: Clinical and radiological data were reviewed for 65 GJT patients treated with GKRS between January 2005 and December 2020 at our center with a mean follow-up period was 87.7 months, and a median was 84 months (range 18 -192 months). The study included 50 females, and 15 males, the median age at presentation was 48 years (range 22–72 years). Five patients were excluded from the study as four did not complete the follow-up criteria, and one died four years post-GKRS because of diabetes mellitus complications. GJTs Patients were deemed eligible for GKRS if the tumor is typically located at the jagulare bulb, contrast enhancement in T1, and fat suppression MRI imaging, less than 4cm in maximum diameters and above the upper border of the second cervical vertebra. Tumors were located on the left side in 36 and the right side in 29 patients. Prior to GKRS, all patients underwent a complete neurological assessment, audiogram, and MRI with contrast examination. The patient's neurological status prior to treatment was used as a reference point. Radiographic studies, including MRI of the brain with different sequences (T1, T2, fat suppression, and T1 with Contrast) and Computed tomography, are infrequently requested.
GKRS was the primary treatment modality in 52 patients (80%), defined tumors by characteristic radiographic neuroimaging, patient history, and neurological examination. GKRS also was used as adjuvant treatment in 13 patients (20%) for residual or recurrent tumors after surgery with pathologic tissue confirmation in 12 patients (4 of them underwent pre-microsurgery embolization). One patient had GKRS for tumor recurrence after fractionated radiotherapy. Table 1
Table I: Summary of 65 GLJTs patients’ characteristics treated with GKRS
Management and gamma knife procedure: All patients were treated using Elekta-Leksell Gamma Knife (models B, and 4-C, depending on the year of treatment); recently, we have used the Icon GKRS model. All cases are treated in a single GKRS session with a frame-based application. The standard Leksell G- stereotactic head frame is applied after local anesthesia application. Frame placement should be shifted toward the tumor side, caudally as much as possible, with the head in flexion position, ensuring easy access to the gamma knife radiation to the whole lesion, avoiding collisions. Target localization was obtained using high-resolution MRI (1.5 Tesla and sometimes the 3 Tesla), obtaining T1, T2, fat suppression sequences, and T1 with contrast at 1.2mm slice thickness on zero angles without a gap. T1-fat suppression and T2 axial sequence were obtained to eliminate tumor edema, bone, and fat. Gamma knife Plans consisted of a mixture of shots depending on tumor volume and the radiation conformity needed. The median tumor volume was 5.4cc (range 1–19.3 cc), the median tumor peripheral prescription dose was 15Gy (12Gy–16Gy), and the median isodose line was 38% (range 35%–60%). The median maximum dose was 42.9Gy (range 31.6Gy–45.7Gy), and the median Lomax conformity index CI Lomax was 0.98 (range 0.89-1). [26] The adjacent area of the brain stem maximum radiation dose was 10Gy or less. Both semicircular apparatus and cochlea received less than 5Gy. Table 2
Treatment was technically feasible for all cases, even for those with low-lying tumors but above the upper border of C2 due to the Low frame placement with the head in flexion as much as possible and using the Open MRI indicator box for MRI neuroimaging.
Table 2: Summary of GKRS treatment parameters for the treated 65 patients with GJTs.
|
Feature |
Median (range) |
|
Initial tumor volume in a cubic centimeter (*cc) |
5.4 (range 1–19.3) |
|
Peripheral prescription dose (PPD) in Gy |
15 (range 12–16) |
|
Prescription isodose line in % |
38 (range 35–60), |
|
Maximal dose in Gy |
42.9 (range 31.6–45.7) |
*cc: Cubic centimeter
Follow-up
Consisted of surveillance, neurological evaluation, and MRI imaging, usually performed six months post-GKRS and then annually for five years, then every two years afterward, or if there were new or worsening symptoms. The mean clinical and radiological follow-up time was 87.7 months (range 18-192 months). The standard GKRS response classification was used to assess treatment outcomes in follow-up, including tumor size control (size unchanged controlled, reduced and regress >10%. or progress) and clinically (unchanged, improved or worsened and additional deficit). MRI sequences of T1, T2, fat suppression, and T1 with Contrast post-GKRS were routinely acquired, and tumor maximum diameters were estimated on 2-D plan MRI images.
Statistics: Continuous features with means, medians, and ranges categorical features were summarized with frequency events and percentages using Excel essential Spreadsheet software. Statistical analyses were performed using MedCalc statistical software package version (20.116). Survival free of radiographic and clinical progression was estimated using the Kaplan-Meier method. The effect of several variables (age, tumor volume, peripheral prescription dose, Lomax conformity index, and pre-GKRS severity of the bulbar symptoms and signs) was evaluated using Cox- proportional hazards regression method.
Literature Search: A systematic literature search on PubMed and Science Direct was performed. The following query terms: "gamma knife" [All Fields] or "stereotactic gamma knife radiosurgery" [All Fields] and "glomus jagulare" [All Fields] or " paraganglioma " [All Fields] and "treatment of GJTs" or "management of GJTs" [All Fields]). There was no time constraint placed on the publication of studies, but studies were limited to those in the English language. We excluded patients with glomus tympanicum and secretory paraganglioma tumors and those treated using Cyber. K and LINAC. Articles included in the study contained patients who had undergone GKRS treatment of glomus jagulare tumor. Articles were excluded if we could not access the complete text, and Case reports were excluded. Crosschecking of the references for relevant articles was performed.
Results
The most common neurological symptoms and deficit at initial evaluation at presentation were pulsatile tinnitus reported in 50 patients. Deterioration of hearing in 46 patients (conductive affection in 42 and sensorineural hearing Loss in 4), and complete hearing loss in 10 patients. Bulbar symptoms, including regurgitation, dysphagia, and dysphonia (IX-X cranial nerves), were detected in 34 patients. Facial nerve palsy was reported in 8 patients (6 of them had post-operative facial nerve palsy and were treated for recurrence and residuals). Tongue deviation and wasting (XII paresis) were noted in 6 patients, shoulder and neck pain in 8 patients, and trigeminal nerve affection was observed in 6 patients. Of the 34 patients who presented with bulbar symptoms, 10 had distressing symptoms, and 24 had mild to moderate symptoms. Most patients had more than one cranial nerve deficit.
Tumor control outcome: The Overall tumor size control rate in this study was 93.8.% (61 patients), 39% (24 patients) showed tumor reduction, and (61%) 37 patients showed unchanged or stable tumor size. Four patients (6.2%) developed tumor progression established in the last MRI images at 62, 68, 84, and 96 months; all clinically worsened or had a new neurological deficit. One patient was re-treated with GKRS after tumor regrowth, another had further fractionated radiotherapy, and the other two did not receive any further surgical or radiation treatment. Tumor progressions post-GKRS in our series were confirmed after five years of follow-up.
In Cox- proportional hazards regression method of different variables (age, tumor volume, peripheral prescription dose, Lomax conformity index CI Lomax), none were significantly correlated with tumor progression-free survival.
The Kaplan-Meier actuarial tumor control rate reported post-GKRS and free of progression was 100% at 3 years, 91.5% at 5 years, and 86% at 10 years of follow-up time. Fig 1.
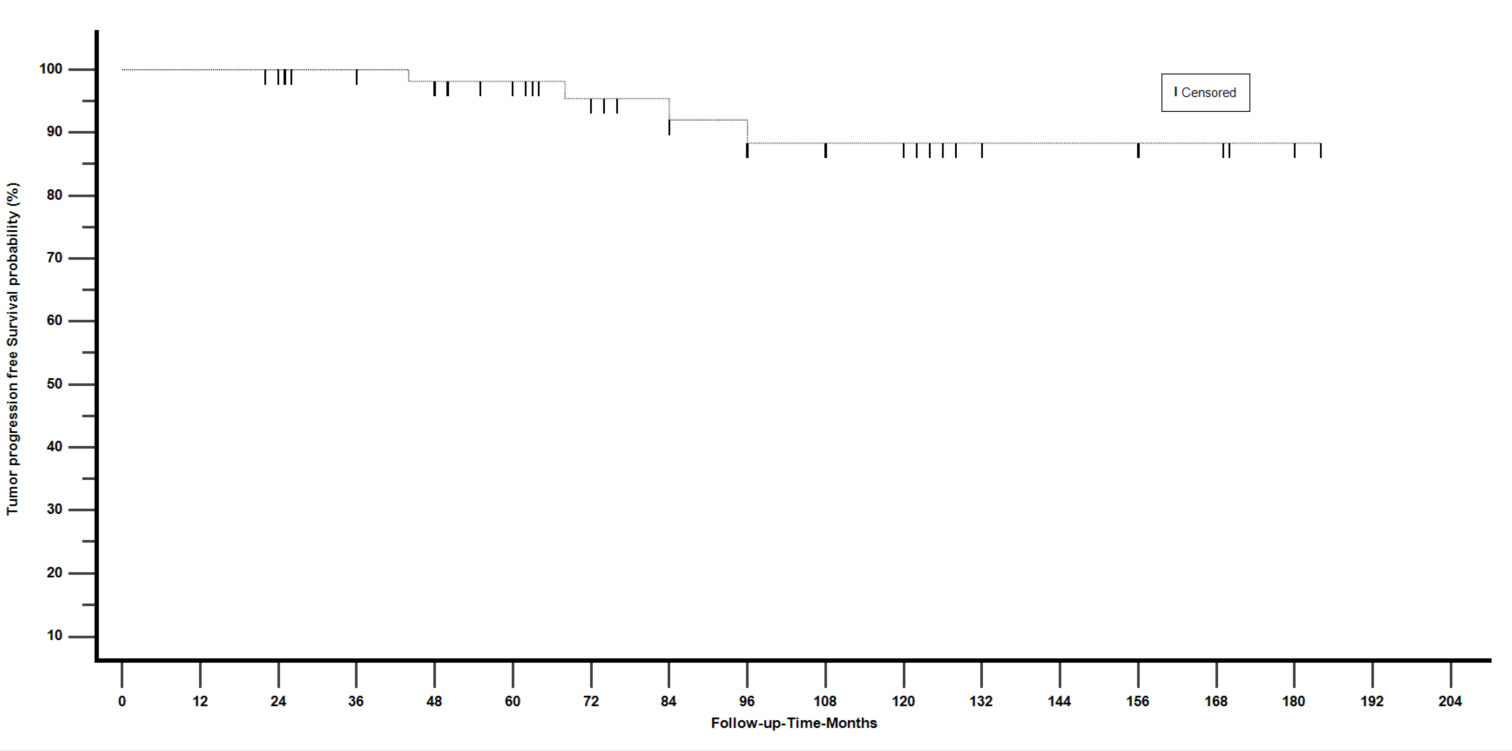
Figure 1: Survival free of radiographic tumor progression using the Kaplan-Meier method.
Clinical outcome: The overall clinical control at the last clinical evaluation was reported in 59 patients (90.8%), with evident improvement in 24 patients (40.7%) and unchanged or clinically stable in 35 patients (59.3%). Although all patients reported improvement of their previous tinnitus, significant improvement was reported in 24/50 patients (49%), and different degrees of bulbar symptom improvement was reported in 18/34 patients (53%). Hearing improvement was noted in 12 patients.
In Cox- proportional hazards regression method with univariate analysis, the severity of distressing bulbar symptoms and signs (IX and X) pre-GKRS were significant predictor factors for the clinical outcome (P=<.0.0194).
In our study, no patient died of side effects related to GKRS, and no adverse radiation reaction was observed.
Complications: New cranial nerve deficits or progression of preexisting symptoms post-GKRS were seen in 6 patients(9.2%). In four of them, there was associated tumor size progression. Clinical progression included a progressive decrease in hearing observed in 4 patients, partial facial nerve palsy in one, progression of bulbar symptoms in 4, and additional trigeminal affection in 2 patients. Severe distressing bulbar symptoms and signs upon presentation were detected in all those six patients. Clinical worsening or additional cranial nerve deficit was reported at a period ranging between 44-108 months post-treatment.
Discussion
Glomus jugulare tumors pose a complex therapeutic challenge because of their location, the usual lower cranial nerve involvement, and the highly vascular nature. Several management options have been described, including surgical removal, endovascular embolization, radiotherapy, and Radiosurgery. The proximity of GJTs to lower cranial nerves, from V through XII, and the hypervascularity elevate the risk of post-operative cranial nerve deficits and propensity for intra-operative bleeding. [7,8,11,16,27,28,29,30] Jackson et al. 2001; in a study of 176 patients with glomus tumors that underwent lateral skull base resections, reported post-operative new Cranial nerve deficit in IX, X, XI, and XII in 39%, 25%, 26%, and 21% of cases, respectively [27]. Ivan and colleagues 2011; conducted a meta-analysis study over 869 GJT patients comparing the morbidity of microsurgery alone, microsurgery with SRS, and SRS alone. The authors reported that patients undergoing SRS alone experienced the lowest rates of recurrence and complications. [29]
The largest multicenter series of the North American Gamma Knife Consortium was reported by Sheehan et al., 2012 [24]. The author observed 132 patients for a median of 50.5 months and found an overall tumor control achieved in 93% of patients; the actuarial tumor control rate was 88% at 5 years post-GKRS, and pulsatile tinnitus improved in 49% of patients. New cranial nerve deficits were noted in 15%. Patel et al., 2019 [1], in a large series of 60 GJT patients treated with GKRS with a mean follow-up of 60 months, reported an overall tumor control rate of 92% and a new cranial nerve deficit of 5%.
In reviewing the literature, we extracted 19 studies that have reported the parameters and outcomes of GKRS treatment for glomus jagulare tumors (GJTs), as shown in Table 3, summarizing the data and outcome of these series. [1, 2, 6, 8, 10, 11, 12, 14, 15, 17, 24, 31, 32, 33, 34, 35, 36, 37, 38]
Table 3: Summary of existing literature series of GKRS treatment for Glomus Jugulare Tumors
|
Author, Year |
Treatment Modality |
Mean Age (Years) |
Number of Patients
|
Mean Follow-up (months) |
Median Tumor Volume*cc |
Median Marginal Dose (Gy) |
Median Maximal Dose (Gy) |
Tumor Control Rate (%) |
Clinical Control Rate (%) |
|
Jordan et al., 2000 [22] |
*GKRS |
61.9 |
8 |
27 |
9.81 |
NR |
33 |
100 |
100 |
|
Saringer et al, 2001 [40] |
GKRS |
63.5 |
13 |
50.4 |
9 |
NR |
NR |
100 |
84.6 |
|
Eustacchio et al, 2002 [7] |
GKRS |
NR |
19 |
86.4 |
5.22 |
14 |
NR |
95 |
94.7 |
|
Bitaraf et al, 2006 [2] |
GKRS |
46.5 |
14 |
18.5 |
9.8 |
18 |
*NR |
100 |
NR |
|
Feigl and Horstmann, 2006 [9] |
GKRS |
51.7 |
12 |
33 |
9.4 |
17 |
NR |
100 |
100 |
|
Gerosa et al, 2006 [13] |
GKRS |
56 |
20 |
50.85 |
7.03 |
17.5 |
NR |
100 |
90 |
|
Sharma et al, 2008 [35] |
GKRS |
46.6 |
10 |
25.4 |
7.9 |
16.3 |
NR |
100 |
100 |
|
Ganz and Abdelkarim, 2009 [16] |
GKRS |
NR |
14 |
28 |
14.2 |
NR |
NR |
100 |
100 |
|
Miller et al, 2009 [28] |
GKRS |
69.6 |
5 |
34 |
4.14 |
15 |
NR |
100 |
100 |
|
Genç et al, 2010 [12] |
GKRS |
50 |
18 |
41.5 |
5.54 |
15 |
NR |
94 |
94.4 |
|
Chen et al, 2010 [3] |
GKRS |
60.1 |
15 |
43.2 |
7.2 |
NR |
NR |
80 |
80 |
|
Navarro Martín et al, 2010 [29] |
GKRS |
56 |
10 |
9.7 |
4.77 |
NR |
29.6 |
100 |
100 |
|
Sheehan et al., 2012 [39] |
GKRS |
58.7 |
132 |
50.5 |
50.5 |
15 |
30 |
92.7 |
85 |
|
Gandía González ML et al, 2014 [11] |
GKRS |
52.4 |
58 |
76.6 |
9.3 |
13.6 |
25.2 |
94.8 |
91.4 |
|
Wakefield et al., 2017 [42] |
GKRS |
64 |
17 |
123 |
9.8 |
15 |
NR |
94 |
94 |
|
Ibrahim et al., 2017 [19] |
GKRS |
55 |
76 |
51.5 |
7 |
18 |
36.7 |
93 |
78.7 |
|
Sharma et al, 2018 [36] |
GKRS |
61 |
38 |
62.3 |
5 |
15 |
28 |
84 |
81 |
|
Patel et al, 2019 [32] |
GKRS |
54.5 |
60 |
66 |
11.6 |
16 |
32 |
92 |
95 |
|
Hellinger RL 2021 [18] |
GKRS |
60 |
29 |
37.3 |
13.9 |
12.8 |
24 |
96.6 |
96 |
|
Current study |
GKRS |
48 |
65
|
87.7 |
5.4 |
15 |
42.9 |
93.8 |
90.8 |
*GKRS= Gamma Knife radiosurgery, *NR= not reported; *cc=Cubic centimeter
Tumor growth control outcome: The overall tumor control rate we obtained was 93.8%. Twenty-four patients achieved tumor size reduction, and 37 had unchanged or stable tumor size. The actuarial tumor size control rates post- GKRS reported were 100% at 3 years, 91.5% at 5 years, and 86% at 10 years.
Our results are comparable to those in the large North American series. The dosimetric parameters reported in Sheehan et al. [24] and Patel et al. [1] series, including tumor volume and radiation doses, are nearly similar to the parameters of our series. Post -GKRS, we did not find a significant correlation between tumor size changes and tumor contrast enhancement, even in the long term. Fig 2

Figure 2: Serial (A) Axial and (B) Coronal contrast MRI brain images for left side 7cc glomus jagulare tumor volume in 44 years old female patient treated with GKRS in 2006 with 15Gy to 35% isodose line. Follow-up images in 2012, 2014, and 2022 showed a gradual decrease in treated tumor size starting in 2014 and became more evident in June 2022.
In the current study, patients who developed tumor size progression post-GKRS (4 pats-6.2%) were reported after the fifth year of follow-up at 62, 68, 84, and 96 months respectively. These findings emphasized the necessity of longer-term follow for such tumors. Series that reported a lower incidence of GJTs tumor progression after GKRS either reported shorter follow-up or was conducted on fewer cases. [6, 17, 31, 34, 35, 36 ] On the other hand, our results are following series reported with more extended follow-up periods that were conducted for a large number of GJT patients. [1, 11, 24, 32, 33, 39]
Clinical outcomes: The overall clinical control at the last clinical evaluation was reported in 59 patients (90.8%), with evident improvement in 24 (40.7%) and unchanged or clinically stable in 35 patients (59.3%). New cranial nerve deficits or worsened pre-treatment deficits were noted in 6 patients (9.2%). The Four patients who developed worsened neurological status or cranial nerve deficits developed in addition to associated tumor size progression confirmed at the fifth year of follow-up. Pulsatile tinnitus significantly improved in 24/50 patients (49%), and bulbar symptoms improved in 18/34 patients (53%). Hearing improvement was observed in 12 patients.
These clinical results corroborated with the findings in many series. [1, 4, 7, 5, 11, 24, 25, 32, 36] The maximum is given dose to the cochlea and the semicircular apparatus in our series was=< less 5Gy.
Ganz et al. [35] reported that clinical improvement was noted 6.5 months after treatment, even though a decrease in imaging was not observed until 13.5 months. Chen et al. [12], in their study of 14 GJT patients treated with GKRS, reported that Clinical and radiologic improvements only sometimes correlated. On the contrary, in the current series, 4 of the six patients with worsening symptoms post-GKRS had radiologically confirmed tumor regrowth. On the other hand, all patients who had tumor size reduction had different degrees of clinical improvement, supporting the relationship between tumor size control and clinical outcomes. These findings support the hypothesis described by many authors that the development of new or worsening cranial nerve deficits could be a predictor sign of tumor growth. [1, 2, 7,11, 25, 36, 37]
Wakefield DV et al. [2], and Dobberpuhl et al. [3], emphasized that Single modality Gamma Knife surgery treatment of glomus jugulare tumors appears safe and efficacious. These findings support our results, where the overall tumor control rate was 98% in 52 patients who received GKRS as primary treatment.
Dharnipragada R. et al. [42], in a meta-analysis, identified 19 studies with a total of 852 GJT patients, 153 patients underwent Radiosurgery, and 699 underwent surgery. The author reported a 3.5% tumor growth rate following Radiosurgery and a 3.9% recurrence rate in surgical resection. The complication rate for Radiosurgery was 7.6% differing significantly from surgical complication rates of 29.6%. These data suggested that Radiosurgery was a reasonable management option for patients with minimal symptoms at high risk for surgery. Furthermore, microsurgical resection should be reserved for patients with lower cranial neuropathies or those who have failed radiation treatment.
The current study, following most of the published series [1, 2, 3, 11, 24, 32, 33, 40, 41, 42] that were conducted on a large number of GJT patients treated with GKRS with long-term follow-up, strengthened the high effectiveness and safety of GKRS in the management of GJTs patients.
Strengthens and limitations: The relative homogeneity of the studied 40 GJTs studied patients strengthens the study in the face of the somehow limited study size of these rare tumors. The mean follow-up period was 87.7 months. This retrospective study represents a limitation. Indeed, single-modality treatment of these tumors depends on multiple factors (size, location, and underlying cranial neuropathies). Considering the slow growth rate of GJTs, Longer-term follow-up of more than ten years and quality of life evaluation warranted further prospective research to assess the effectiveness and safety of GKS as a primary mode of treatment for these tumors.
Conclusions
Our experience stands and supports most of the published series regarding the established role of GKRS as a highly effective tool for most GJT patients' treatment with a tumor control rate of 93.8% at an extended mean follow-up of 87.7 months with a low rate of morbidity. Among the Several management options for GJT patients, documented GKRS favorable outcomes are very challenging and even comparable to microsurgery results. Therefore GKRS could be safely and effectively considered a first-line management option for most GJTs patients, excluding extensive giant tumors or those with an extension below the C2 vertebra.
Retrospective study: For this study, formal consent is not required; it does not contain any studies with human participants.
Funding: No funding was received for this research.
Competing interests: The authors declare that they have no competing interests and certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial in the matter or materials discussed in this manuscript. We declare that this is an original article.
Acknowledgments: We want to thank Dr. Tiit Rahn, M.D., Ph.D. for long-term assistance in many patients evaluation and management, Dr. Mahmoud El Badrawy for assistance in dose calibration, treatment dose conformity and patient management, and we extremely grateful to Mrs. Hadeer Ezz for her sincere efforts in through patients direct communications and collection of patients paper and electronic data