Neurosurgery and Neurology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2025
ISSN No: 2836-2829 | Journal DOI: 10.61148/2836-2829/NNR
Kesar Prajapati1, Jaya Pathak2, Parth Adrejiya3, Malay Rathod4
1Senior Resident Doctor, Baroda Medical College, Department of Medicine, Vadodara- 390001, Gujarat, India.
2Associate professor, Baroda Medical College, Department of Medicine, Vadodara- 390001, Gujarat, India.
3Baroda Medical College, Vadodara- 390001, Gujarat, India.
4Baroda Medical College, Vadodara- 390001, Gujarat, India
*Corresponding author: Kesar Prajapati, Senior Resident Doctor, Baroda Medical College, Department of Medicine, Vadodara- 390001, Gujarat, India.
Received date : September 07, 2022
Accepted date : September 21, 2022
published date : September 26, 2022
Citation: Prajapati K, Pathak J, Adrejiya P, Rathod M, (2022) “How to obtain a peaceful brain to save the Ukraine! Help Joe, Ole and Jane to save the Ukraine!”. J Neurosurgery and Neurology Research, 4(2); DOI: http;//doi.org/011.2022/1.1043.
Copyright: © 2022 Kesar Prajapati. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background- Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) is a hereditary cerebral arteriopathy caused by mutations in the NOTCH-3 gene on the short arm of chromosome 19, that encodes the NOTCH-3 receptor protein, predominantly expressed in adults by vascular smooth muscle cells and pericytes. It commonly presents with stroke, migraine with aura, cognitive impairment, acute encephalopathy, and psychiatric disturbances.
Case presentation- Here we report a case of a 48-year-old female with repeated episodes of stroke, a history of forgetfulness, and behavioral changes. MRI showed subacute infarcts, multiple foci of increased signal intensity on T2, and some microhemorrhages. Genetic testing identified Notch 3 gene thus confirming the diagnosis.
Conclusion- CADASIL might be an underestimated cause of recurrent stroke and should be considered in the differential diagnosis. In this paper, we describe an overview of etiology, pathogenesis, clinical presentation, investigations, and treatment of CADASIL.
Main Text
Background: CADASIL(Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy) causes a type of stroke and dementia whose key features include recurrent subcortical ischemic events and vascular dementia [1]. CADASIL is the most common heritable cause of stroke and vascular dementia in adults with equal sex distribution having a mutation in the NOTCH3 gene on chromosome 19q12 [2]. It may present with migraine with an aura, psychiatric disturbances and mood disorders in the elderly, and progressive cognitive impairment. It is a very rare and invariably fatal disease, estimated to affect 2/100,000 adults based on several epidemiological studies, including one performed in Scotland (with similar rates all around the world), although it is thought to be widely underdiagnosed [3]. Magnetic resonance imaging (MRI) of CADASIL patients shows characteristic periventricular white matter hyperintensity in T2 weighted images in asymptomatic CADASIL patients [4]. Later T1-weighted images disclose multiple lacunar infarcts in white matter and deep grey matter, the volume of which correlates with disability [5]. The presence of Granular Osmiophilic Material (GOM) in capillary blood vessels of the skin and muscle on biopsy and genetic studies (Notch 3 analysis) plays a key diagnostic role in that Notch 3 testing has been proposed as the primary diagnostic approach, allowing the detection of 90% of affected individuals [6].
Case Presentation: A 48-year-old, female presented with four days history of acute onset right upper and lower limb weakness after waking up from sleep, which was nonprogressive. There was no loss of consciousness, no history of headache, vomiting, convulsion, diplopia, blurring of vision, difficulty in swallowing, chest pain, palpitation, and head injury. According to the patient's relative, she also had a lack of social interaction, behavioral changes, and forgetting things kept by her for 6 months. In the last 5 years, the patient has had four similar attacks of stroke. The first episode of right-sided hemiparesis happened five years ago which was recovered completely over a period of one month, the second episode of right side upper limb was four years back, recovered completely over a period of three months, two years, and one year back third and the fourth episode of right-sided hemiparesis happened respectively and was recovered completely over a period of three to four months. Family history suggests that her mother was having similar complaints and behavioral changes and died at age of 65 years. There was no history of hypertension and diabetes mellitus and the use of oral contraceptive pills or any other medications. On examination, pulse was 100 beats/min and blood pressure was 150/90 mmHg and speech was normal. The CNS examination showed that the patient was conscious, depressive mood, and had a loss of recent memory. Gait was short steppage with reduced ground clearance, increased tone with hyperreflexia in the right side upper and lower limbs with positive Babinski sign. Cardiovascular and Respiratory system examinations were unremarkable.
The patient's routine hematological investigations were unremarkable with normal biochemistry, clotting tests, and renal function tests. The patient’s 12 lead ECG, chest x-ray, and 2D ECHO were normal. Optic fundoscopy was normal. Mini-Mental State Examination (MMSE) is a 30-point point questionnaire that provides measures of orientation, registration (immediate memory), short term memory as well as language functioning. Our patient’s score was 19. Frontal Assessment Battery (FAB) is a brief tool at the bedside or in a clinic to assist in discriminating between Dementias with frontal dysexecutive phenotype and Dementia of Alzheimer's Type (DAT). The total score is a maximum of 18, a higher score indicates better performance. Our patient’s score was 9. MRI brain showed increased signal intensity on Diffusion-Weighted Imaging (DWI) in the left thalamic and left the periventricular region with low Apparent Diffusion Coefficient (ADC) suggestive of the subacute infarct. Multiple foci of increased signal intensity on T2 FLAIR are seen in the bilateral cerebral white matter, subcortical white matter, and left cerebellum suggestive of an old ischemic lesion. Few foci of blooming are seen in the bilateral frontal region likely microhemorrhages. These findings suggest small vessel ischemic disease. MRI is shown in Figure 1.
Figure 1. MRI of the patient demonstrated Multiple foci of increased signal intensity on T2 FLAIR are seen in the bilateral cerebral white matter, subcortical white matter, left thalamic and left periventricular region.
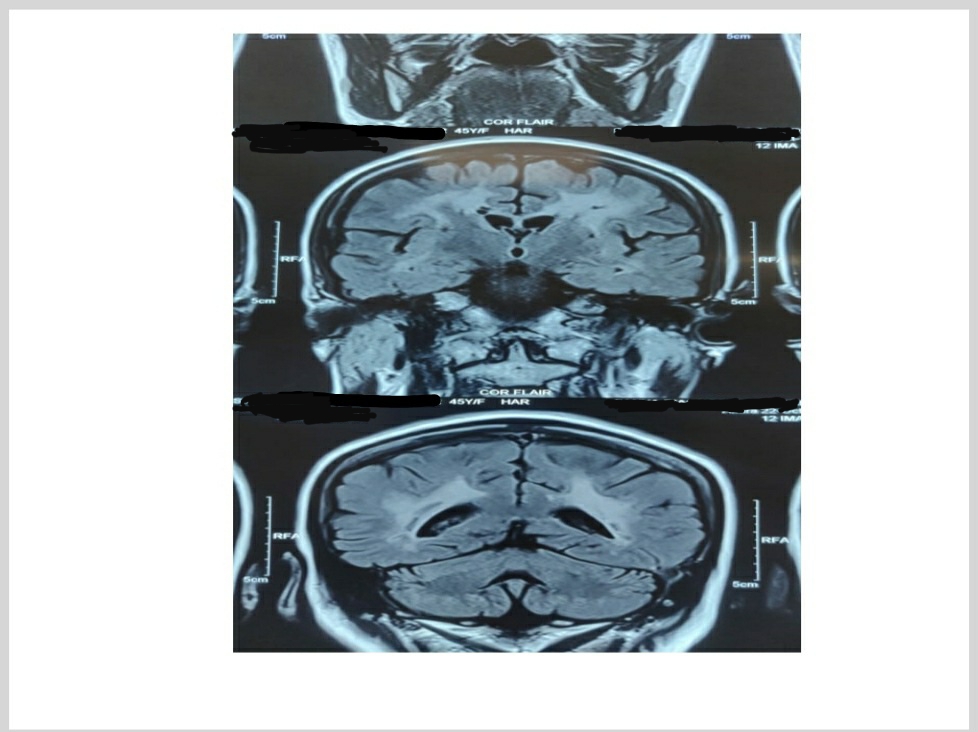
A lumbar puncture showed no evidence of oligoclonal IgG in serum or cerebrospinal fluid (CSF) and CSF: IgG albumin ratio was normal (0.6) and CSF glucose and protein were within the normal range. Her secondary causes of hypertension were ruled out. An ultrasound carotid Doppler showed normal carotid artery velocities bilateral with normal cephalad flow within both vertebral arteries. There was no surgically significant stenosis.
As a recurrent episode of hemiparesis, It includes thromboembolic stroke,mitochondrial encephalopathy and stroke-like episodes (MELAS), migraine disorders, multiple sclerosis, vasculitis, Moya Moya disease, and Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL/CARASIL). She was put on Aspirin 75 mg/day, Atorvastatin 40 mg/day, and Amlodipine 10 mg/day and kept on follow-up. Genetic analysis was carried out and Notch 3 gene mutation on chromosome 19 had been detected which confirmed the diagnosis of CADASIL. She was referred for genetic counseling and was kept on regular follow-up.
Discussion:
CADASIL first reported by Van Bogaert as ‘hereditary Binswanger’s disease’ in 1995, is the most common genetic cause of ischemic stroke [7]. In the UK, its prevalence appears to be about 2 per 100 000 but this may be an underestimate as the disorder is thought to be misdiagnosed [3]. The clinical phenotype later observed in French families became known as CADASIL. A major breakthrough was achieved in 1993 when Tournier-Lasserve mapped the disease gene locus to chromosome 19 using a positional cloning approach [6]. The mean age of onset is 35-45 years and the disease tends to follow a stepwise deterioration but it can be insidious [12].
An acute encephalopathy occurs in 10% of cases. CADASIL has received attention in the neurological literature but there remains a relative dearth of reporting in clinical and academic psychiatry [3]. Stroke in a young patient is multifactorial and cardioembolic risk factors such as congenital heart disease including a patent foramen ovale, mitral valve prolapse, and an atrial septal aneurysm should be considered. Rheumatic fever, infective endocarditis, systemic lupus erythematosus, and antiphospholipid syndrome should also be considered. Polyarteritis nodosa, Behcet’s syndrome, sarcoidosis, and primary central nervous system angiitis are less common causes. Syphilis, tuberculosis, borreliosis, and HIV are infections that can present as strokes. Sickle cell disease, primary vasculitis, and hypercoagulable states may also present as a stroke. The white matter signal abnormality is nonspecific and seen in many diseases. However, the involvement of the anterior temporal lobes (86%) and external capsules (93%) is specific to suggest the diagnosis of CADASIL in appropriate clinical settings [8,9].
CADASIL is rare and its clinical features are nonspecific, and it is almost never considered a leading differential at first presentation. MRI is relatively specific in the early course of the disease and helps to differentiate between Mitochondrial Encephalopathy and Stroke Like Episodes (MELAS), migraine disorders, and demyelinating diseases like multiple sclerosis. Early involvement of the anterior temporal lobes and external capsules is characteristic in the initial stages of CADASIL. As the disease progresses there is the involvement of the white matter which can also be seen with advanced demyelinating disease and microangiopathy. After observing such findings, one should do a genetic analysis [10]. It suggests that different subcortical areas have different vulnerabilities to ischemia in CADASIL[4]. The diagnosis is confirmed by sequencing the NOTCH3 gene. The pathological hallmark of CADASIL is the deposition of GOM in close relation to vascular smooth muscle cells. It can also be detected in vessels of extracerebral tissues including skin and muscle; therefore skin biopsies can also be used for diagnosis. Specificity is high (approaching 100%), but sensitivity is only 50%. Electron Microscopy (EM) reveals GOM deposits in vascular smooth muscle cells [12]. Increased susceptibility to cortical spreading depression may be a possible mechanism for an increased aura prevalence. Atypical auras, such as prolonged visual auras, gastrointestinal manifestations, dysarthria, confusion, and focal neurological defects can occur. Transient ischemic attacks and stroke occur in 85% of symptomatic individuals. Its clinical phenotype may involve presenting as a classic lacunar syndrome but other ischemic syndromes (brainstem or hemispheric) are also observed.
Simple focal seizures propagating towards the medial temporal lobe, pseudobulbar palsy, and urinary incontinence may also occur [14].
Vascular risk factors should be addressed such as weight reduction, exercise, smoking cessation, and reduced alcohol intake. Antiplatelet therapy with a combination of Aspirin, Dipyridamole, or Clopidogrel is regarded as optimal prophylaxis against further thromboembolic stroke. Aggressive management of hypertension and the inclusion of a statin is also very important. Homocysteine levels are elevated in CADASIL, treatment with folic acid is also important. Optimal management of comorbidities and strict glycemic control is also required. By the time of death which occurs at a mean age of 61 years, approximately 75% of patients are fully dependent on carers[13].
In this case of CADASIL, the patient had a recurrent attack of ischemic stroke with behavioral changes and a family history consistent with similar complaints in her mother who died at the age of 65. Then the MRI was done which showed on T2 FLAIR increased signal intensity in bilateral cerebral white matter, subcortical white matter, and left cerebellum suggestive of the old ischemic lesion and few foci of blooming are seen in the bilateral frontal region likely microhemorrhages. CSF was done to rule out other differentials like infections, and multiple sclerosis. With strong suspicion of Cerebral Autosomal Dominant Arteriopathy genetic analysis of NOTCH 3 gene mutation was carried out and came positive in the present case. Changes were evident in global performance (MMSE), language, and memory. CADASIL involves a mutation in the NOTCH3 gene on chromosome 19q12 [12]. Notch 3 is a 2321 amino acid type I transmembrane protein, which is believed to be involved in the specification of cell fate during development [10].
The main marker of disease progression is increasing age. The overall course is highly variable even within single families. Some patients remain asymptomatic until their 70s, whereas others are severely disabled by the age of 50s. Early onset does not necessarily predict rapid progression. There is no definitive treatment for CADASIL. In the absence of a curative approach, treatment should be directed toward the search for possible disease-modifying strategies to mitigate clinical manifestations [15].
Informed Consent- Written consent was obtained from the patient.
Consent of Ethics- Ethical approval is not required at our institution to publish an anonymous case report.
Conflict of interest- The authors declare that there is no conflict of interest regarding the publication of this case report.
Financial support- Funding- None
Acknowledgment- N/A