Neurosurgery and Neurology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2025
ISSN No: 2836-2829 | Journal DOI: 10.61148/2836-2829/NNR
Ahmed Hosameldin *, Ahmed Owis, Mohammed Abdellatif Hussein, Mostafa Abdel-latif
Department of Neurosurgery, Fayoum University Hospitals, Fayoum University, Fayoum, Egypt
*Corresponding Author: Ahmed Hosameldin, Department of Neurosurgery, Fayoum University Hospitals, Fayoum University, Fayoum, Egypt.
Received date : June 22, 2022
Accepted date : June 30, 2022
published date : July 11, 2022
Citation: Hosameldin A, Owis A, Mohammed A Hussein, Mostafa Abdel-latif, (2022) “Influence of Inner Membranectomy after Evacuation of Chronic Subdural Hematoma: Recurrence and Recollection Rates”. J Neurosurgery and Neurology Research, 4(2); DOI: http;//doi.org/011.2022/1.1042.
Copyright: © 2022 Ahmed Hosameldin. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Chronic subdural hematomas (cSDH) is one of the most commonly encountered neurological disorders in the neurosurgery practice. Despite most symptomatic patients are being treated by surgical drainage, the optimal surgical technique for treating cSDH, till present, remains unclear and as to whether or not membranectomy should be performed. Therefore, we conducted this study to compare the surgical outcomes in patients undergoing cSDH drainage with and without inner membranectomy.
Methods: A prospective comparative study was conducted on 80 patients in need of surgical evacuation of cSDH during the period from January 2018 to September 2021. They were divided into two equal groups: group 1 undergoing evacuation with partial inner membranectomy and the other group without membranectomy.
Results: The mean conscious level within each group improved significantly (P = 0.05), where it increased from 12.2 to 14.6 in group 1 and from 12.4 to 14.5 in group 2 in the postoperative period. The mean motor power increased significantly (P = 0.001) in group 1 from 2.1 to 4.6 and in group 2 from 2.2 to 4.5, postoperatively. Pneumoncephalus was the most commonly anticipated postoperative complication with an occurrence rate of 37.5% in group 1 and 62.5% in group 2, however, they resolved spontaneously with no surgical intervention. There was no recurrence of cSDH in group 1 while four patients in group 2 had recurrent cSDH. No mortality occurred in our series.
Conclusions: Burr-hole craniostomy with partial inner membranectomy is a preferable approach in terms of surgical outcomes of better motor power, better conscious level, less recurrence rate, and shortened hospital stay.
Introduction
Chronic subdural hematoma (cSDH), a frequently presenting neurological disease in neurosurgery departments, is readily diagnosed by non-contrast computed tomography (CT) scans.[11] The reported incidence of cSDH in the literature is approximately 14 per 100, 000 per year, and is rising appreciably in the elderly population, [2, 13] as they are 20 times more likely to present with cSDH.[7, 14] The use of oral antiplatelet and anticoagulant agents is believed to play a role in the high incidence of cSDH associated with trivial trauma in elderly. The pathology of this condition was claimed to multiple theories but the commonest was tearing of bridging veins suspended to visceral surface of dura. [8, 14, 18] Such condition is managed variably, however, there is a consistent consensus about the preferred approach for symptomatic patients which is surgical drainage. That being said, the ideal surgical approach remains controversial.[3] The most commonly used surgical procedures for treatment of cSDH vary from bedside twist drill craniostomy or single or multiple burr hole drainage to craniotomy with drain insertion, irrigation, and/or membranectomy (i.e. resection of the subdural inner and/or outer membranes). [6, 19] Recently, burr hole drainage showed superiority over the other two techniques in terms of lower rates of recurrence and morbidity.[14, 19] However, there is no data of a direct
comparison between each of these approaches to conclude the optimal surgical approach.[22] On the other hand, many authors recommend resection of all capsular membrane components (i.e. outer and inner membranes), allowing for brain re-expansion and reduction in the postoperative potential subdural space.[1-21] At present, the surgical management of cSDH remain unclear as to whether or not membranectomy should be performed. Moreover, it has been reported that postoperative convulsions in cSDH patients with either partial or complete membranectomy occurs variably; therefore, this maybe a long-term complication of this management option.[20] On the other hand, the long-term repercussions of not removing the membrane remain unclear, so the potential impact of the membrane warrants further investigation. Therefore, we conducted this study to compare the outcomes of burr-hole evacuation of cSDH in two sets of patients: with partial membranectomy and without membranectomy, in terms of re-operation due to recollection or recurrence, complications rates, clinical outcomes in the form of assessment of conscious level and motor power immediate postoperative and for three month follow up.
Methods
Between January 2018 to September 2021, 80 patients with CSDH who were indicated for surgical management, were prospectively included in a prospective randomized comparative study. CSDH was defined as a subdural hematoma with a surrounding capsule (hematoma membrane) which consists of dark reddish liquefied blood at the time of operation and proved by CT/MRI. All patients, of both genders, above 40 years of age, with CSDH irrespective of the underlying etiology who were confirmed by either CT or MRI, presenting to our neurosurgery team during the study period and were operated upon for the first time, were included in our study. Asymptomatic patients with thin film CSDH, patients with recurrent CSDH after previous operation or with calcified hematomas were excluded. All eligible patients were asked for participation and a written consent was received from each individual willing to participate or from direct relative prior to conducting the study. A thorough history taking and clinical examination was done for all enrolled patients. In all cases CT brain was used for diagnosis of CSDH and post-operative assessment. On the other hand, MRI was performed for a specified set of patients to define the multi-compartment nature of the hematoma and to allow for detailed visualization of the hematoma membranes. Recruited patients were categorized into two groups: Group 1 consisted of 40 patients who were operated upon via Burr-hole craniostomy (BHC), evacuation and inner membranectomy and eventually drainage into a closed drainage system; Group 2 consisted of 40 patients who were operated upon via BHC, evacuation and then drainage into a closed drainage system without inner membranectomy.
Surgical technique: Operations were performed under general anesthesia for all patients. Two burr-holes were made then expanded to coin size using kerrison rongeur and the dura was cauterized using bipolar cautery and then opened via a cruciate-manner incision. Afterwards, subdural cavity was irrigated using warm normal saline until the color of the fluid became clear. The inner dural membrane was lifted by a forceps and 3 to 5 cm were torn. Just after the irrigation, the tip of a nelton catheter was inserted at the posterior parietal burr-hole and the proximal tip of the catheter was directed fronto-temporal 5 to 7 cm and then was left in the subdural space. Eventually, subdural space was filled with physiological normal saline and was taken into a closed drainage system. The drain was removed from the posterior parietal burr-hole and fixed at a level approximately 10 cm below the patients head. About 36 to 72 hours postoperatively, the drain was removed. The only exception in Group 2 is that they did not undergo membranectomy procedure and only passed through traditional burr hole evacuation technique. Antibiotics, analgesics, gastro-protective agents, intravenous fluid, neurotonic drugs and prophylactic anti-epileptic drugs were routinely administered to all patients. However, patients presenting with motor deficits were referred to physiotherapy for 3 months or till improvement of motor function to normal. All patients were followed up for a variable period of time, during which full neurological examination was performed to detect any improvement or deterioration in the neurological condition of the patients. Moreover, CT was re-performed in the 1st and 3rd postoperative day to assess re-expansion of the brain, reduction in the subdural space thickness, improvement of midline shift, incidence of pneumoencephalus, recollection or reperfusion injuries. The drain was removed 36 hours to 72 hours after surgery. Eventually, patients were discharged upon stabilization of their condition, with regular follow up till three month in outpatient clinic to assess recurrence of clinical signs. A CT brain was done to detect any recollection of the hematoma at 1 and 3 months postoperative.
Statistical Analysis
Data were collected, coded, and entered into Microsoft Access. Data analysis was performed using Statistical Package of Social Science (SPSS-Version 21). The mean and standard deviation (SD) of assessed variables were presented. Categorical variables were expressed as numbers and percentages. Independent t-test was used to compare the measures of two independent groups of quantitative data. Paired t-test was used for the purpose of comparing two groups of qualitative data. Chi square test (χ2) was used to compare more than two groups. Mc-Nemar test was used for pained dependent qualitative variables. P value of ≤ 0.05 was considered the cut-off point for statistical significance
Results
A total of 80 patients were included in the final analysis: 40 patients in group 1 and 40 patients in group 2. Group 1 consisted of 28 male and 12 female patients with an age ranging from 55 years to 87 years (mean: 70.5 ± 11.6). Group 2 consisted of 32 male and 8 female patients with ages varying between 57 years and 82 years (mean: 67.2 ± 8.74). Patients characteristics are presented in Table 1.
|
Variables |
Technique |
p-value |
|
|
|
|
With |
without |
|
|
|
Age (mean in |
70.5 |
67.2 |
>0.05 |
NS |
|
Classifcation according to different age groups (No. & %) |
||||
|
<60 years old |
4 (10%) |
12 (30%) |
>0.05 |
NS |
|
60–75 years old |
28 (70%) |
20 (50%) |
>0.05 |
NS |
|
>75 years old |
8 (20%) |
8 (20%) |
>0.05 |
NS |
|
Sex |
|
|
|
|
|
Male |
28 (70%) |
32 (80%) |
>0.05 |
NS |
|
Female |
12 (30%) |
8 (20%) |
>0.05 |
NS |
Table 1: Comparison between demographic characters in different study groups.
Preoperative assessment revealed that 24 patients in Group 1 had a history of mild or moderate head trauma, whereas 28 patients in Group 2 had a history of head trauma. On the other hand, history of coagulopathy was identified in 16 patients in Group 1 and 8 patients in Group 2, indicating preoperative need for correction of their coagulopathy. Our analysis revealed that 28 patients in Group 1 scored more than 12 on the GCS while 12 scored less than 12, whereas Group 2 showed nearly the same results with 30 patients scored more than 12. None of the patients in Group 1 had history of epileptic seizures, whereas only one patient in Group 2 reported a history of epileptic seizures. Sixteen patients in Group 1 had speech affection, whereas twelve patients in Group 2 complained of speech difficulties. Concerning the preoperative motor power, eight
patients in Group 1 had grade 0, four patients had grade 1, twelve had grade 2, eight had grade 3, eight had grade 4, and none of the patients had grade 5. As regards Group 2, sex patients had grade 0, eight had grade 1, eight had grade 2, seven had grade 3, seven had grade 4, and four patients had grade 5. There was no statistically significant difference (P-value >0.05) in terms of conscious level (GCS), history of fits, speech affection and motor power. Upon analyzing the radiological findings, we found that in Group 1, twenty patients had left-sided hematoma; sixteen patients had right-sided hematoma; four patients had bilateral hematoma. On the other hand, in group 2, twenty patients had left-sided hematoma; twelve had right-sided hematoma; eight had bilateral hematoma. From the perspective of density of hematomas, in Group 1, eight patients had hypodense, sixteen had isodense, and sixteen patients had mixed-density hematomas. On the other hand, in group 2, eight patients had hypodense, twenty had isodense, and twelve patients had mixed-density hematomas. No statistical significant difference was noted (P-value >0.05) as regards both the location and density of hematomas. Based on the Markwalder grading system for CSDH, Group 1 had none with grade 1, while sixteen patients had grade 4 upon hospital admission. On the other hand, Group 2 had four patient with grade 1 and none with grade 4. None of the patients of both groups had grade 0 as all patients in our population were symptomatic, requiring surgical drainage. Preoperative assessment revealed that 50% of patients in group 1 and 40% of patients in group 2 were classified as good. Postoperative assessment revealed similar results in both groups where all patients were classified as good. Data regarding Markwalder pre- and post-operative assessment are presented in Table 2.
|
Markwalder |
Technique |
Sig. |
|
|
With |
Without |
||
|
No. (% ) |
No. (% ) |
||
|
Preoperative |
|||
|
Grade 0 |
0 (0%) |
0 (0%) |
>0.05 NS |
|
Grade 1 |
0 (0%) |
4 (10%) |
>0.05 NS |
|
Grade 2 |
20 (50%) |
12 (30%) |
>0.05 NS |
|
Good |
20 (50%) |
16 (40%) |
>0.05 NS |
|
Grade 3 |
20 (50%) |
24 (60%) |
>0.05 NS |
|
Grade 4 |
0 (0%) |
0 ( 0% ) |
>0.05 NS |
|
Bad |
20 (50%) |
24 (60%) |
>0.05 NS |
Table 2: Comparison of pre- and post-operative evaluation in different study groups according to Markwalderts grading system for CSDH.
Assessment of the thickness of subdural space of recruited patients was performed three times: preoperative, postoperative, 1 and 3 months after discharge. Regarding preoperative thickness of the hematoma in Group 1, the average was 23 ± 6 mm (range: 14-37 mm), whereas for group 2 the average thickness recorded was 21.5 ± 6.49 mm (range: 15-34). During the postoperative time, we detected an average thickness for group 1 of 8. 5 ± 6.1 mm (range: 5- 22 mm), whereas for group two, the average thickness was 12 ± 6.3 mm (range: 4-24 mm). At the follow up period (three months after discharge), group 1 showed an average thickness of 2 ± 2.5 mm (range: 0-8 mm), whereas, the thickness for group 2 was 6.1 ± 6.3 mm (range: 3-18 mm). Such differences between the two groups at the 1 month follow up period were statistically significant (P-value =0.05). In terms of midline shift in our population, average preoperative shift in group 1 was 10 ± 4.2 mm (range: 0–15 mm) and 10.3 ± 4.2 mm (range: 0–13 mm) for group 2; average postoperative shift was 4 ± 2.2 mm (range: 0 – 6.5 mm) for group 1 and 5.1 ± 3.15 mm (range: 0 – 9 mm) for group 2; average shift after one month was 0.24 ± 0.44 mm (range: 0 - 1 mm) for group 1 and 0.84 ± 0.88 mm (range: 0 - 2 mm) for group 2. The differences in midline shift between the two groups after one month are shown to be of statistical significance (P-value =0.05). Our analysis revealed significant improvement in conscious level (GCS) in both study groups (P-value <0.05). The mean GCS in group 1 increased from 12.2 ± 2.7 in the preoperative period to
14.5 ± 0.85 on the 3rd postoperative day, whereas for group 2 the mean GCS increased from 12.4 ± 2.9 in the preoperative period to 14.4 ± 0.84 on the 3rd postoperative day. In the same context, we detected a highly significant improvement in motor power (MP) as regards both study groups (P-value =0.001). The mean MP in group 1 increased from 2.1±1.4 in the preoperative period to 4.5 ± 0.5 one month after surgery, whereas for group 2 the mean MP increased from 2.3±1.9 in the preoperative period to 4.5 ± 0.5 one month after surgery. As for postoperative complications, 30% of patients in group 1 and 20% of patients in group 2 suffered from seizures. In the same context, 37.5% of patients in group 1 and 62.5% of patients in group 2 suffered from pneumoencephalus. However, none of these patients needed tapping or reoperation as pneumoencephalus appeared to be resolving spontaneously. Surprisingly, none of the patients in group 1 suffered from recurrence of CSDH, whereas 20% of patients in group 2 showed recurrence of CSDH. Two of the eight patients who had recurrent hematoma were clinically intact with no midline shift and were managed conservatively till spontaneous absorption, however, another patient presented with recurrent hemiparesis and was re-operated upon by craniotomy, evacuation of clotted hematoma and excision of membranes. No cases of mortality occurred in both study groups. The differences in complication rates in both groups are statistically insignificant (P-value >0.05) (Table 3).
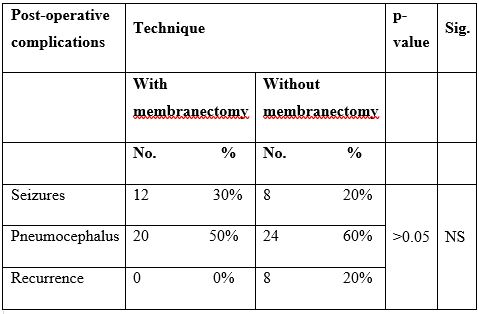
Table 3: Comparisons of post-operative complications in different study groups.
In terms of postoperative hospital stay, group 2 had longer average hospital stay compared to group 1 (4.5 ± 1.7 vs 4.1 ± 1.6 days). However, the difference is of no statistical significance (P-value >0.05).
Discussion
Considered as one of the most common neurological conditions; cSDH is a frequent disorder in the elderly population, usually following a minor head trauma as well as in patients on long term antiplatelet or anticoagulant medications. Patients on anticoagulant or antiplatelet therapy tend to present with bilateral subdural hematomas. We are comparing the surgical outcomes of evacuating cSDH in patients operated upon by two slight different technical approaches: evacuation with partial inner membranectomy and evacuation without membranectomy. To the best of our knowledge, the postoperative findings of inner membrane tearing, to date, has only been discussed in a single study performed by Kayaci et al and have not been discussed elsewhere in the literature.[11] The male-to-female ratio of cSDH incidence in our study was 2.3/1 in Group 1 and 4/1 in Group 2, revealing a male predominance, however, this finding is insignificant. This finding goes in line with Kayaci et al. who reported a similar ratio of 4.3/1 in the group undergoing inner membrane tearing.[11] This could be related to the large cranium of men with severe atrophy in the old age leading to easy separation of the dural border cell layer, explaining the high prevalence in men. On the other hand, cSDH is hard to develop in females unless cranial asymmetry is great. [16] In our study, cSDH showed a peak at the age group of 60 to 75 years old, going in line with Kayaci et al. results.[11] Age distribution in both study groups were similar. This could be attributable to the degree of cerebral atrophy occurring as people age, leading to decrease in brain tissue with increase in cerebrospinal fluid and venous fragility, predisposing to the occurrence of cSDH.[15, 16] A history of minor or moderate head trauma is reported to be the frequent presentation in symptomatic cSDH patients.[11] 65% of our population presented with a history of head trauma. Noteworthy, 30% of our patients had a history of coagulopathy which was in need of correction prior to surgery. This may be related to the high prevalence of liver disease in Egypt which in turn negatively impact the process of coagulation and platelet functionality.[5] In this study, we detected similar results regarding the side of the cSDH in both groups where half of the patients in both study groups had left sided cSDH. Bilateral cSDH occurred in four patient in group 1 and in eight patients in group 2. These results are quite similar to Kayaci et al. findings. As for the density of cSDH in our population, isodense hematoma was the most prevalent density followed by mixed density and hypodense hematoma, respectively.[11] No cases of hyperdense hematoma were detected in our population. We used MRI to assess the density of cSDH, as it has shown superiority over CT in terms of detection of isodense cSDH and small clots near the skull base and vertex and also accurate estimation of the age of cSDH.[9] Regarding midline shift, group 1 had a noticeable reduction in the average midline shift from the preoperative period to the 1st and 3rd postoperative day to one month after surgery. Interestingly, the degree of reduction was quite similar in both groups. However, statistical significance was only noted in the difference in average midline shift one month after surgery. This goes in contrast with the findings of Kayaci, however, he detected that the shift diminished more clearly in the inner membranectomy group compared to the non-membranectomy group.[11] Patients with cSDH were scored using both GCS and Markwalder grading systems in order to evaluate the severity of injury and to compare the outcomes before and after the surgical approach. The level of consciousness (GCS), in our study, improved significantly within each group where the mean score increased at a similar pattern in both groups from the preoperative evaluation to the postoperative evaluation. The study groups were matched as regards conscious level upon recruiting patients where twenty-eight patients in group 1 and thirty in group 2 scored more than 12 while twelve patients in group 1 and ten patients in group 2 scored less than 12 on GSC scale. This finding is relatively similar to the results of Kayaci et al as significant improvement on GSC was noted in the inner membranectomy group. As for Malkwalder grading system, 50% of patients in group 1 were classified as good and 40% of patients in group 2 were classified as good in the preoperative period. Postoperative assessment showed that all patients in both groups were classified as good. Similarly, both groups showed highly significant improvement in the motor power within each group, one month after surgery. The mean hospital stay in our study was 4.1 ± 1.6 days for group 1 and 4.5 ± 1.7 days for group 2. However, it was relatively higher in the study groups of Kayaci et al.[11] Such differences could be attributed to the pre-existing medical conditions and co-morbidities in Kayaci et al. population which in fact led to higher mean hospital stay. In terms of postoperative complications, none of the patients in group 1 had recurrence of cSDH while eight patients in group 2 showed recurrence of symptomatic cSDH. No mortalities were recorded in our population. Recurrence of cSDH is a commonly faced complication which has been studied by many neurosurgeons and its pathogenesis remains debatable. Both mortality and recurrence are commonly caused by accompanying medical conditions or complications of the poor clinical status of the patients preoperatively rather than by surgical incompetence complications. Re-bleeding in the outer membrane of the hematoma is the most widely accepted theory so far.[12] However, five major risk factors play a highly contributable role in its occurrence after surgery, which are hematoma density, presence of postoperative intracranial air, location of the catheter tip, intracranial hematoma extension, and hematoma width or thickness.[10] Poor brain re-expansion capability is also proposed to a leading cause in the occurrence of recurrence of cSDH.[6] Interestingly, none of the membranectomy group in our study showed any signs of recurrence. Many studies demonstrated the importance of outer and inner hematoma membranes in recurrence. Putnam and Cushing proposed the beneficial outcomes of membranectomy in preventing recurrence, depending on the belief that by manipulating the inner membrane, the impact of these membranes that act as barriers to brain re-expansion were also removed.[17] Moreover, a recent meta-analysis of the outcomes of membranectomy in cSDH demonstrated a reduction in recurrence rates on average in patients undergoing membranectomy compared to alternative interventions such as craniotomy or
burr-hole drainage without membranectomy, while exhibiting similar morbidity and mortality profile as other surgical approaches reported in the literature.[19] Pneumoencephalus was a frequent postoperative complication estimating a rate of 37.5% in patients of group 1 and 62.5% of patients in group 2. This is comparable to the rate reported in Zakaraia et al. study which was estimated in 40% of patients.[23] Kayaci et al reported similar rates, however, the rate was less in the membranectomy group due to better brain re-expansion.[11] Excessive pneumocephalus in their population was dealt with by tapping and eventually showed improvement. On the other hand, none of our patients were in need of tapping or reoperation as pneumocephalus appeared to be resolving spontaneously. Our study has several limitations, the most important is the small sample size in each group, making our findings non-generalizable and in need for further assessment. Also, the preexisting medical conditions were not thoroughly assessed which could have affected the outcome endpoints in any possible way.
Conclusion:
Surgical evacuation of cSDH through burr-hole and partial inner membranectomy is a preferable method in the treatment of cSDH in terms of lower recurrence rates, better postoperative motor power and conscious level, and less pneumocephalus incidence, compared to evacuation without membranectomy.