Neurosurgery and Neurology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2025
ISSN No: 2836-2829 | Journal DOI: 10.61148/2836-2829/NNR
Ahmed Hosameldin
Department of Neurosurgery, Fayoum University Hospitals, Fayoum University, Fayoum, Egypt.
Corresponding author: Ahmed Hosameldin, Department of Neurosurgery, Fayoum University Hospitals, Fayoum University, Fayoum, Egypt.
Received date : December 29, 2021
Accepted date : January 19, 2022
published date : January 24, 2022
Citation: Ahmed Hosameldin, (2022) “Endoscopic Third Ventriculostomy for Obstructive Hydrocephalus in Children; Challenges and Clinical Outcomes”. J Neurosurgery and Neurology Research, 4(1); DOI: http;//doi.org/011.2022/1.1037.
Copyright: © 2022 Ahmed Hosameldin. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Background: Endoscopic third ventriculostomy (ETV) has been established as a viable treatment option for obstructive hydrocephalus of children over 8 weeks of age. ETV in pediatric groups may be unsuccessful due to the failure of redirection of cerebrospinal fluid (CSF) flow, re-closure of ventriculostomy opening or due to infection. The exact cause is still debatable. Some issues like failure to eliminate the second membrane during the procedure or formation of the new arachnoid membrane at the stoma are still not clear. This study aims to assess the surgical failure of ETV and its predisposing factors.
Methods: Thirty-two pediatric patients with hydrocephalus were analyzed retrospectively to assess efficacy of endoscopic third ventriculostomy in children. The patients’ age limit was between 6 months and 12 years. This is a retrospective study of 32 patients in Fayoum University Hospital in the period between May 2017 and December 2020. Patients having hydrocephalus in pediatric groups more than 8 weeks of age were included in the study.
Results: The mean age of all patients was 24 months and the mean follow-up period was six months. Of 32 ETVs, the success rate was 78% in 25 patients and the failure rate was 22% in 7 patients. The study included 24 males (75 %) and 8 females (25%) with a male to female ratio (3:1). Clinical presentations varied from enlarged head (macrocrania), dilated scalp veins, repeated vomiting and poor ocular fixation and following. Complications were divided into failure of procedure, infection, CSF leak and re-exploration.
Conclusions: Endoscopic ventriculocisternostomy remains an effective surgical technique in the treatment of obstructive hydrocephalus. It is linked to a very low rate of permanent morbidity and avoids ventriculo- peritoneal shunt-related morbidity and long life shunt dependence. But we should take in consideration good selection of indicated cases especially in pediatric groub.
Introduction
Non communicating hydrocephalus either congenital, secondary to infection, hemorrhage or an obstructive lesion [3,11,21]. This pathology, which is frequent in the pediatric environment, recognizes a multitude of etiologies, most often malformative and haemorrhagic in the neonatal period, post-meningitis in infants and tumours in older children [24,25,27]. For a long time, cerebrospinal fluid (CSF) bypass shunts were the only treatment for hydrocephalus. The development of neuro-endoscopy has brought other possibilities in the treatment of this pathology. Endoscopic third ventriculostomy (ETV) is now considered a vital treatment option for obstructive hydrocephalus. ETV has been established for many children with hydrocephalus as an effective treatment [16]. This lack of data collected for efficacy of ETV in children limits our ability to answer questions regarding ETV complications and efficacy. Precise data on intraoperative events are especially lacking, and the effect these events can have on ETV performance. While the ETV Performance Score (ETVSS) has helped surgeons predict the performance of the operation based on preoperative factors, identifying significant intraoperative factors can further assist decision-making surgeons and perhaps provide insight into the critical technical elements of an optimal ETV [9,13,18,20]. The diagnosis of shunt malfunction was made based on clinical symptoms and radiologic signs, including ventricular dilatation on CT. Some CT scans showed only slight differences in ventricular dimensions compared with previous radiologic studies performed when the shunts were functioning, reflecting the loss of ependymal elasticity and brain compliance in these patients after a long-term shunt [5,6]
Methods
Data were collected from children who underwent ETV for obstructive hydrocephalus in pediatric groups from 6 months to 12 years between May 2017 and December 2020. This is a retrospective study of 32 patients. Most patients had undergone recent magnetic resonance imaging (MRI) of the brain for routine ETV in hydrocephalus patients. In emergency cases, a CT scan of the brain was done. Before doing re-exploration for ETV, MRI was considered in symptomatic patients. The selection criteria of patients for ETV were based on clinical symptoms and signs and the CT scan of the brain or MRI of the brain in pediatric hydrocephalus group
who were more than 6 weeks old. The parameters studied included clinical (tri ventricular dilatation, tetra ventricular dilatation); etiological (congenital, infectious, tumor); endoscopic findings (hemosiderin deposits, choroid plexus atrophy or hyperplasia, pulsation of the 3rd ventricle floor, arachnoid adhesions, hypothalamic adhesions, closure of the aqueduct of Sylvius, narrow retroclival space, venous bleeding ).
Outcome assessment
Clinical outcomes were assessed using neurological examination and Glasgow Coma Scale (GCS). Also CSF leak was assessed daily till removal of stitches. Day after day sterile dressing is recommended to avoid infection. While radiological assessment was through serial CT scans on 1st day, one week and one month postoperative. In some cases MRI postoperative was required with CSF flowmetry study to assess the difference in dynamics of CSF flow. All patients were followed up monthly for six months period in outpatient clinic. All statistical analyses were performed using SPSS software version 21. The p value < 0.05 was deemed significant.
Surgical Technique
All procedures with patients under general anesthesia and in a supine positionhave been performed, and the little LOTTA system of KARL STORZ was used for all cases which are autoclave compatible. After a linear incision (2–3 cm) of the skin, an approach was made via a Kocher frontal burr hole (1–2 cm in front of the coronal suture and 2.5 cm lateral to the midline) to ensure an adequate trajectory. Foramen of monro was identified and the endoscope was advanced to the third ventricle. (Figure 1)

Figure 1: Endoscopic view of Foramen of Monro
The pre-mesencephalic cistern was inspected and in between infundibular recess and mammillary bodies in the midline a fenestration was made through the monopolar electrode and was enlarged further via Fogarty balloon catheter for sufficient CSF flow. The endoscope was advanced to the stoma for inspection of the basilar artery and its branches and also to look for the other membranes in the pre-pontine space. (Figure 2) (Figure 3)
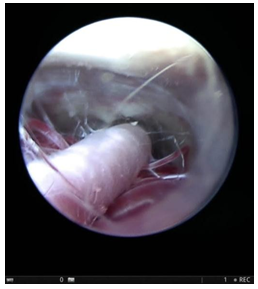
Figure 2: Endoscopic view through ventriculostomy stoma showing Basilar artery and its branches
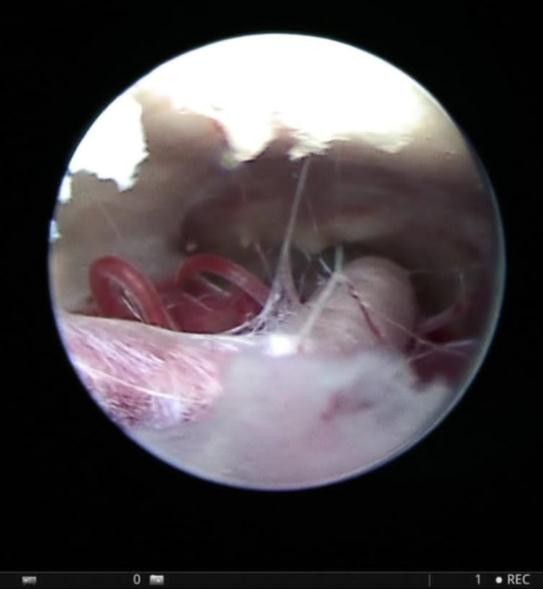
Figure 3: Endoscopic view through ventriculostomy stoma shows membranes in the pre-pontine space
Results
The present study included a total of 32 patients. Among them, 24 (75%) were male and 8 (25%) were female patients. We included patients between 6 months to 12 years in our study. The mean age of all patients was
32.25 ± 35.90 months (range 6–144 months). The age range from 6 to 36 months was the most affected (84.4%) with a male predominance (sex ratio of 3:1). Table 1.
|
Variable |
Number (%) |
|
Sex |
|
|
Males |
24 (75%) |
|
Females |
8 (25%) |
|
Age |
|
|
8 to 36 months |
27 (84.4%) |
|
36 months and above |
5 (16.6%) |
Table 1: Sex and age in our study
Etiology varied through patients, twenty-six patients (81.25%) had congenital anomaly or malformation of ventricular system mostly aqueduct of Sylvius stenosis , four patients (12.5%) infectious, two patients (6.25%) tumoral. Table 2.
|
Etiology |
Number (%) |
|
Congenital |
26 (81.25%) |
|
6 to 36 months |
4 (12.5%%) |
|
36 months and above |
2 (6.25%%) |
Table 2: Etiology variations
Clinical manifestations varied from enlarged head, bulging anterior fontanelle, repeated vomiting, poor suckling, poor crying, seizures, dilated scalp veins and visual disturbance especially poor fixation or following in infants. Table 3.
|
Clinical manifestations |
Number (%) |
|
Enlarged head |
32 (100%) |
|
Bulging anterior fontanelle |
28 (87.5%%) |
|
Repeated vomiting |
20 (62.5%%) |
|
Poor suckling |
12 (37.5%) |
|
Poor crying |
8 (25%) |
|
Seizures |
12 (37.5%) |
|
Dilated scalp veins |
4 (12.5%) |
|
Visual disturbance |
6 (18.75%) |
|
Sunset appearance |
2 (6.25%) |
|
Delayed developmental milestones |
20 (62.5%%) |
Table 3: Clinical manifestations
Neuroimaging characteristic of obstructive hydrocephalus, represented by the ventricular dilatations. The average duration of the procedure was 40 minutes. In our study, there were no intraoperative complications of endoscopic procedures. The endoscopic finding revealed in almost all patient aqueductal stenosis, a choroid plexus hyperplasia in 4 patients (12.5%) for whom choroid plexus coagulation was done, multiple thin thread like membranes in 4 patients (12.5%) which denoted signs of infection, 2 patients (6.25%) showed hemosiderin deposits indicating hemorrhagic course and narrow retroclival space were observed in 16 patients (50%). In this series, all patients were followed for at least 6 months. During follow-up, patients were examined regularly at outpatient clinic to assess the head size, developmental milestones like neck holding, sitting, and walking were evaluated along with psychomotor assessment. Two patients (6.25%) revealed a delay in developmental milestones. A postoperative CT scan of the brain was performed postoperative at 1st day, one week, one month and six months routinely. Any patient who had increased size of the head, tense anterior fontanelle, or any sign of raised intracranial pressure with evidence of increased ventricular size in CT was advised for MRI of the brain. Post-operatively, there were twenty six patients (81.25%) without complications, while six other patients (18.75%) had complications in the form of CSF leak in 4 patients (12.5%), superficial wound infection in 2 patients (6.25%), failure of procedure and re-exploration with shunt insertion in one patient (3.1%).
Discussion
Obstructive hydrocephalus is a difficult challenge to many neurosurgeons for precise decision making and several studies have examined the different treatment options and their effectiveness [14,19,29]. ETV has rising reputation in the treatment of obstructive hydrocephalus rather than traditional ventriculo-peritoneal shunting procedures in selected patients. ETV has been used for children with obstructive hydrocephalus as an effective measure of treatment. Some authors in literature documented predisposing factors to failure of ETV especially in pediatric group. Although there is lack of data considering its efficacy and complication rates. In our study, we discuss causes and challenges of pre-operative decision making to identify factors predictive of ETV dysfunction in cases of obstructive hydrocephalus in children. We conducted a study on 32 children with obstructive hydrocephalus in which precise decision was made to perform an ETV to divert CSF flow through a newly formed stoma to overwhelm the obstructive pathology. In or study, we found a male predominance of (75%) with a sex ratio (M/F) of 3:1. Several studies have shown a predominance of males and this predominance could be linked to the fact that congenital hydrocephalus can be transmitted in a sex-linked recessive mode [19,29]. The age of the children ranged from 6 months to 144 months (12 years) with a mean of 32.25 ± 35.90 months. The age range from 8 to 36 months was the most affected (75%). Ignorance and lack of knowledge of parents may result in delay of seeking medical consultation. According to clinical signs progressive macrocephaly followed by bulging anterior fontanelle and dilated scalp veins respectively. As regards to age, the incidence of ETV dysfunction is higher in infants than in children [3]. According to Kadrian et al., there is a strong effect of patient age on outcome [1]. Drake and colleagues concluded that the success rate of ETV essentially depends on patients age. [7]. A recent study observed that younger patients with preterm birth had a lower success rate comparing those patients who were mature at the birth time [17]. According to Heshmati, ETV’s success rate was around 60% which is lower than the recorded rates in pediatric populations [15]. In our study, we got a 75% ETV success rate in all patients. Within two years, the success rate was 60% while above two years success rate was 90%. Feng et al. found that ETV successfully treated obstructive hydrocephalus in 75% of patients [10]. Duru et al. documented their experience with ETV in 51 children under the age of 16; they reported an overall success rate of 80% for all etiologies and ages [8]. According to the report of Sodhia et al., the success of ETV in children aged 1–2 years also amounted to 80% [23]. Salvador S. F. et al. found in 2014 that age is a predictive factor of endoscopic ventriculo-cisternostomy dysfunction [22]. The etiological diagnosis of hydrocephalus was based on patient history, clinical findings, and CT brain. In our study, congenital hydrocephalus due to aqueductal stenosis was the most common cause and consanguineous marriage could be charged for such predominance. Other causes were addressed such as infection, hemorrhage and tumors. Vaessen S. et Coll in Liege in 2006 reported in their study of hydrocephalus in children that malformations were the most frequent cause followed by idiopathic malformations with proportions of 35% and 15% respectively [28]. In a study, the univariate and multivariate analysis showed that both hydrocephalus etiology and patient age were relevant factors predicting ETV success [12]. The complication rate is widely recognized as being linked to the surgeon’s experience. In our study, Leakage of CSF through the incision was reported in 6 cases and only one case of wound infection that was managed with antibiotics and repeated dressings. Failure of ETV was encountered in 8 patients (25%) in our series. Those patients were re explored endoscopically to assess the cause of failure and we found that stoma closure was encountered in two patients, newly formed arachnoid membranes either pre-mesencephalic or over ventriculostomy was encountered in three patients, blood clot was also in charge of 3rd ventricular floor obstruction in one case and finally two cases with no obvious cause of failure. Bouras T. et al. in his meta- analysis claimed that CSF leakage through the operative wound is the most frequent postoperative complication found in the literature; according to, the incidence of this complication varies between 0-5.2% with an average of 1.7% [4]. We believe that thin skin and immature subarachnoid spaces in pediatrics could be related to this result. Baldauf J. et al. mentioned that the existence of arachnoid adhesions in patients shows a higher proportion of ETV failure compared to the absence of this abnormality [3]. The output of an ETV presents possible risks like CSF leakage, infection, subdural hygromas, and hematomas. ETV has rescued the hydrocephalic patient from shunt dependency and its complications as a foreign body [15]. Yadav et al. mentioned that pre-operative identification of the exact etiology of hydrocephalus can increase the success rate of the ETV and avoid unnecessary surgery [30]. So we agree that the correct selection of patients is essential for achieving good results with ETV. Previous shunting operation and complex hydrocephalus were reported to be the main causes of ETV failure [12]. So, we can delineate predisposing factors that cause ETV failure are ventriculostomy stoma closure by new arachnoid granulation tissues, second membrane formation within the stoma or pre- mesencephalic , CSF absorption failure, CSF infection/high protein, and improper selection of patients. The mechanism of failure usually in other studies is the closure of the stoma due to local inflammatory reaction, and its incidence is also related to the underlying pathology.
Conclusion
The endoscopic third ventriculostomy, in general, remains an effective surgical technique in the treatment of obstructive hydrocephalus and could be very effective method with precisely selected pediatric age group. It has the upper hand as it avoids ventriculo-peritoneal shunt-related morbidity or being lifelong shunt dependent. Factors causing ETV failure are ventriculostomy stoma closure by new arachnoid membranes, remnant or newly formed pre-mesencephalic membranes, infection or high protein content.