Neurosurgery and Neurology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2025
ISSN No: 2836-2829 | Journal DOI: 10.61148/2836-2829/NNR
Abdulrahman K. Alanezi 1*, Faisal T. Sayer 1
1 Department of Neurosurgery, Ibn Sina Hospital, Ministry of Health, Kuwait City, Kuwait
*Corresponding Author: Abdulrahman K. Alanezi, M.D, Neurosurgery Resident
IBN SINA HOSPITAL (Department of Neurosurgery), P.O. Box 25427, Safat – 13115, State of Kuwait
Received date : November 22, 2021
Accepted date : November 29, 2021
published date : December 02, 2021
Citation: Abdulrahman K. Alanezi, Faisal T. Sayer. “Fungal Brain abscesses and Severe Rapidly Progressive Guillain-Barré Syndrome in the Setting of Acute Covid-19 Disease”. J Neurosurgery and Neurology Research, 3(1); DOI: http;//doi.org/011.2021/1.1033.
Copyright: © 2021 Abdulrahman K. Alanezi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The world has been facing an outbreak of the novel coronavirus (COVID-19) that originated from Wuhan, China since December 2019 and this outbreak caused a rapidly spreading pandemic of the severe acute respiratory syndrome coronavirus 2 (sars-cov-2). Typically, patient with COVID-19 experience respiratory symptoms; however, a wide range of other symptoms has been described. In this report, we present a 74 years old male with typical clinical and electrophysiological manifestations of Guillain-Barré syndrome (GBS) three weeks after discharge from the hospital due to COVID-19 infection associated with bilateral fungal brain abscesses.
Introduction
COVID-19 is a new disease that is caused by the severe acute respiratory distress syndrome–coronavirus‐2 (SARS‐CoV‐2), which is commonly manifesting as pneumonia and potentially could result in acute respiratory distress syndrome (ARDS) that requires admission to the intensive care.(1)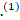 However, a wide variety of neurological symptoms were reported including headache, conscious level impairment, anosmia, loss of taste and seizures,(1)(2)
However, a wide variety of neurological symptoms were reported including headache, conscious level impairment, anosmia, loss of taste and seizures,(1)(2)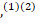 with multiple reports indicating an emergent of neurological illnesses include encephalitis;(2)(3)
with multiple reports indicating an emergent of neurological illnesses include encephalitis;(2)(3)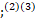 and strokes.(3)(4)
and strokes.(3)(4) In addition, a link between COVID-19 and Guillain-Barré syndrome (GBS) was recognized in multiple case reports and one case series of five patients.(1)(5-10)
In addition, a link between COVID-19 and Guillain-Barré syndrome (GBS) was recognized in multiple case reports and one case series of five patients.(1)(5-10)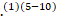
Case Presentation and Clinical Course
On 30th of June 2020, a 75 years old gentleman known to have hypertension and chronic lymphocytic leukaemia (CLL) (in remission), presented to the hospital with two days history of fever and loss of appetite associated with mild cough and dizziness. There was no headache, no vomiting, no limbs weakness or other neurological symptoms. On examination, the patient was conscious, alert and oriented. Clinical examination and vital signs were within normal. His lab results showed WBC 54.8, Hb 77, Plt 233, lymphocyte 35.6 CRP 68, LDH 1506, PCT 0.40, Na 136, K 3.5, Urea 11.7, Creat. 125. Chest X-ray showed bilateral lung infiltration. Nasopharyngeal swab for COVID-19 was positive. Patient was isolated for Ten days in a COVID-19 ward, then transferred to a medical ward, where he stayed for 20 days as per advice the of haematology team in relation to his CLL, then discharged home in good general condition.
Two weeks after discharge, the patient presented again with severe acute onset of dysarthria and bilateral lower limb weakness. On examination, he had a mild form of dysarthria and power was assessed as 4/5 (MRC scale) in both lower limbs. MRI brain showed small bilateral high parietal ring-enhancing lesions with surrounding vasogenic oedema highly suggestive of abscesses. This was managed conservatively with triple antibiotics therapy including vancomycin 1g BD, ceftriaxone 2g BD, metronidazole 500mg TDS along with amphotericin B 50mg OD. Follow-up MRI brain, ten days later, due to clinical deterioration, showed progression in the size of the brain abbesses, more for the left side lesion (Figure 1).
|
|
|
|
|
A |
B |
C |
Figure 1: MRI Brain with contrast T1 (A-B) & T2 (C)
From that point on, the neurosurgery team was involved in the care of the patient. Neurological examination revealed that the patient was conscious, opening eye spontaneously and obeying simple commands with no verbal response (Glasgow Coma Score of 10/15). There was a right-side facial nerve palsy. For the lower limbs, there was a symmetrical weakness (motor power 0/5), hypotonia with absent reflexes at both ankles and knees. For the upper limbs, there was asymmetrical weakness, with motor power (0/5) for the right UL and (2/5) for the left UL except for left-hand grip where power was 4/5. Right Upper limb was hypotonic, while left Upper limb showed normal tone but absent reflexes of both brachioradialis and biceps. Urgent MRI cervical spine, to rule out an epidural collection that could explain the clinical presentation, was within normal. Due to the size of the left side abscess and close proximity to the lateral ventricle, the patient underwent urgent evacuation of the left side abscess through a parietal burr hole and needle aspiration using brain lab navigation. About 15 ml of thick greyish and blood-tinged fluid was aspirated.
A few days later and due to our impression that the abscesses cannot fully explain the clinical condition of the patient, electromyography (EMG) was performed and the findings supported motor and sensory peripheral neuropathy, mainly axonal with elements of demyelination suggesting an axonal Guillain-Barré syndrome rather than critical illness neuropathy (Tables 1&2), which is a variant of GBS.
|
MNCS |
||||||
|
Nerve |
CMAP |
Amplitude |
Distance |
CV |
F-M Lat |
|
|
Onset (ms) |
Duration (ms) |
mV |
mm |
m/s |
ms |
|
|
Fibular – Peroneal Motor Left |
||||||
|
Ankle- EDB |
No compound muscle action potential |
|||||
|
Fibular – Peroneal Motor Right |
||||||
|
Ankle- EDB |
3.87 |
9.0 |
0.77 |
|
|
Absent |
|
Bl.knee-Ankle |
10.7 |
11.0 |
0.41 |
310 |
45.4 |
|
|
Ab.knee-Bl.knee |
13.0 |
13.1 |
0.39 |
70 |
30.4 |
|
|
Peroneal – TA Motor Left |
||||||
|
Be.Fibular Head - - |
3.74 |
9.3 |
0.20 |
|
|
|
|
Peroneal – TA Motor Right |
||||||
|
Be.Fibular Head - - |
3.38 |
15.8 |
0.66 |
|
|
|
|
Lat.Popliteal fossa-Be.Fibular Head |
5.52 |
12.8 |
0.63 |
70.0 |
32.7 |
|
|
Post.tibial Motor Left |
||||||
|
Med.ankle-Abd. hal |
6.03 |
10.0 |
1.72 |
|
|
49.8 |
|
Median Motor Left |
||||||
|
Wrist-APB |
2.96 |
7.6 |
3.6 |
|
|
|
|
Elbow-Wrist |
7.25 |
7.2 |
2.9 |
240 |
55.9 |
|
|
Axilla-Elbow |
8.88 |
11.6 |
2.6 |
|
|
|
|
Erb’s-Axilla |
13.3 |
8.5 |
2.0 |
|
|
|
|
Median Motor Right |
||||||
|
Wrist-APB |
3.20 |
9.5 |
0.68 |
|
|
Absent |
|
Elbow-Wrist |
7.69 |
10.9 |
0.54 |
240 |
53.5 |
|
|
Axilla-Elbow |
10.4 |
13.1 |
0.61 |
|
|
|
|
Erb’s-Axilla |
13.3 |
14.6 |
0.58 |
|
|
|
|
Ulnar Motor Right |
||||||
|
Wrist-ADM |
2.90 |
10.5 |
0.90 |
|
|
Absent |
|
Bl.elbow-Wrist |
6.71 |
12.2 |
0.36 |
240 |
63.0 |
|
|
Ab.elbow-Bl.elbow |
8.58 |
13.5 |
0.40 |
100 |
53.5 |
|
|
Axilla-Ab.elbow |
10.8 |
14.9 |
0.39 |
|
|
|
|
Erb’s-Axilla |
13.6 |
12.3 |
0.13 |
|
|
|
Table 1: Motor Nerve Conduction Studies
|
SNCS |
|||||
|
Nerve |
Start Lat |
PeakLat |
Amp |
Distance |
CV |
|
ms |
ms |
uV |
mm |
m/s |
|
|
Suralis Sensory Right |
|||||
|
Mid. Lower leg – Lat. Malleolus |
3.09 |
3.20 |
9.3 |
130 |
42.1 |
|
Median Sensory Left |
|||||
|
Wrist – Dig II |
3.06 |
3.70 |
14.5 |
140 |
45.8 |
|
Median Sensory Right |
|||||
|
Wrist – Dig II |
2.91 |
3.52 |
19.6 |
140 |
48.1 |
|
Ulnar Sensory Right |
|||||
|
Wrist – Dig V |
2.41 |
2.79 |
25.7 |
140 |
49.0 |
Table 2: Sensory Nerve Conduction Studies
In addition, the patient has no history of ICU admission; therefore, critical illness neuropathy was not considered as the definitive diagnosis. The patient was seen by the neurologist who recommended five doses of IVIG (30g X 5 days), then to repeat EMG after 14 days of the 1st dose of IVIG. 14 days later, electromyography (EMG) was repeated again and showed the presence of significant proximal conduction block with drop of compound muscle action potential and sensory nerve action potential supporting our initial diagnosis which is axonal Guillain-Barré syndrome (Tables 3&4).
|
MNCS |
||||||
|
Nerve |
CMAP |
Amplitude |
Distance |
CV |
F-M Lat |
|
|
Onset (ms) |
Duration (ms) |
mV |
mm |
m/s |
ms |
|
|
Fibular – Peroneal Motor Left |
||||||
|
Ankle- EDB |
No compound muscle action potential |
|||||
|
Fibular – Peroneal Motor Right |
||||||
|
Ankle- EDB |
3.85 |
9.8 |
0.42 |
|
|
Absent |
|
Bl.knee-Ankle |
10.7 |
10.5 |
0.13 |
310 |
45.3 |
|
|
Ab.knee-Bl.knee |
13.2 |
12.0 |
0.11 |
70 |
28.0 |
|
|
Peroneal – TA Motor Right |
||||||
|
Be.Fibular Head - - |
2.96 |
13.0 |
0.89 |
|
|
|
|
Lat.Popliteal fossa-Be.Fibular Head |
4.77 |
18 |
0.41 |
70.0 |
38.7 |
|
|
Post.tibial Motor Left |
||||||
|
Med.ankle-Abd. hal |
5.23 |
20.4 |
0.24 |
|
|
Absent |
|
Post.tibial Motor Right |
||||||
|
Med.ankle-Abd. hal |
5.55 |
9.5 |
0.90 |
|
|
|
|
Pop.fossa-med.ankle |
13.1 |
12.0 |
0.32 |
350 |
46.4 |
|
|
Median Motor Right |
||||||
|
Wrist-APB |
2.84 |
12.2 |
0.34 |
|
|
Absent |
|
Elbow-Wrist |
8.27 |
14.6 |
0.26 |
240 |
44.2 |
|
|
Erb’s-Elbow |
13.8 |
14.4 |
0.22 |
|
|
|
|
Ulnar Motor Right |
||||||
|
Wrist-ADM |
2.77 |
9.5 |
0.48 |
|
|
Absent |
|
Bl.elbow-Wrist |
7.00 |
10.5 |
0.16 |
240 |
63.0 |
|
|
Ab.elbow-Bl.elbow |
8.73 |
11.7 |
0.16 |
230 |
54.4 |
|
|
Erb’s-Axilla |
13.6 |
16.1 |
0.13 |
|
|
|
Table 3: Motor Nerve Conduction Studies
|
SNCS |
|||||
|
Nerve |
Start Lat |
PeakLat |
Amp |
Distance |
CV |
|
ms |
ms |
uV |
mm |
m/s |
|
|
Suralis Sensory Right |
|||||
|
Mid. Lower leg – Lat. Malleolus |
2.96 |
3.53 |
6.4 |
125 |
42.2 |
|
Radialis Sensory Right |
|||||
|
EPL tendon – Wrist |
1.48 |
1.90 |
20.4 |
80.0 |
54.1 |
|
Median Sensory Right |
|||||
|
Wrist – Dig II |
2.76 |
3.46 |
24.9 |
140 |
50.7 |
|
Ulnar Sensory Right |
|||||
|
Wrist – Dig V |
2.32 |
2.90 |
21.2 |
120 |
51.7 |
Table 4: Sensory Nerve Conduction Studies
All the lab results from the aspirated fluid were negative, apart from showing pus cells. A week later, we repeated the CT head, which showed no change in the size of the brain abscesses. The patient underwent bilateral parietal craniotomy with pus evacuation and excision of the lesions (thick capsule), which was sent for histopathology, and was positive for fungal abscesses with angioinvasion. Patient started on IV Voriconazole (anti-fungal medication) for three weeks.
Few days later, during the patient hospital stay, he developed an episode of desaturation with a saturation of 89% spo2, where he required high flow oxygen. Chest X-ray was done and showed diffuse lung infiltration (Figure 2).
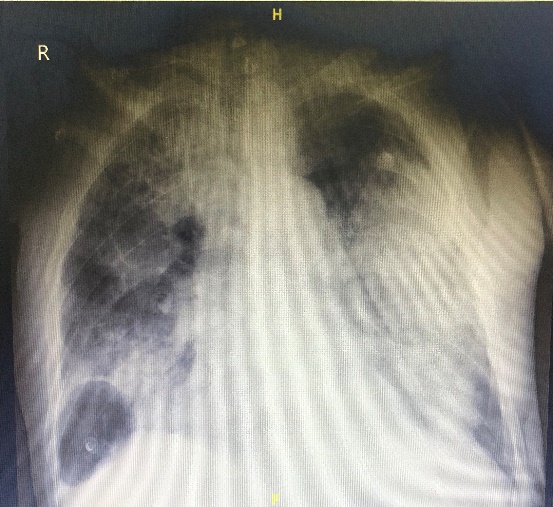
Figure 2: Chest X-ray
Nasopharyngeal swab for COVID-19 was taken and confirmed a positive result. Patient was isolated and admitted to the ICU, where he deceased.
Discussion
Physician in China reported the first case of GBS post COVID-19 infection,(1) followed by case series of five adult patients in Italy.(10)
followed by case series of five adult patients in Italy.(10) One case in Iran ,(7)
One case in Iran ,(7) two cases in the UK.(5)(6)
two cases in the UK.(5)(6) , one case in the US.(9)
, one case in the US.(9) and one paediatric case in Saudi Arabia.(8)
and one paediatric case in Saudi Arabia.(8)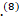 In this report, we describe an association between GBS and COVID-19 infection in a 74 years old male, who had confirmed COVID-19 infection and bilateral frontal brain abscesses.
In this report, we describe an association between GBS and COVID-19 infection in a 74 years old male, who had confirmed COVID-19 infection and bilateral frontal brain abscesses.
Several mechanisms have been postulated through which SARS-Cov-2 enters the CNS, causing neurological complications such as acute cerebrovascular disease, meningitis, encephalitis and GBS .(19)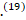 These include blood circulation pathway, neuronal pathway by anterograde and retrograde transport, immune-mediated injury by Cytokine Storm Syndrome (CSS) and direct infection injury to the CNS through the cribriform plate to the brain.
These include blood circulation pathway, neuronal pathway by anterograde and retrograde transport, immune-mediated injury by Cytokine Storm Syndrome (CSS) and direct infection injury to the CNS through the cribriform plate to the brain.
GBS is a heterogeneous condition with several variants that manifest as an acute inflammatory polyradiculoneuropathy resulting in diminished reflexes and weakness elicited by a preceding infection.(11)(16)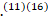 The believed theory behind it is that there is an immune response to a preceding infection cross-reacting with peripheral nerve components because of molecular mimicry. The immune response can be directed towards the myelin or the axon of peripheral nerves, resulting in demyelinating and axonal forms of GBS. The most commonly identified precipitant of GBS is Campylobacter jejuni infection.(11)
The believed theory behind it is that there is an immune response to a preceding infection cross-reacting with peripheral nerve components because of molecular mimicry. The immune response can be directed towards the myelin or the axon of peripheral nerves, resulting in demyelinating and axonal forms of GBS. The most commonly identified precipitant of GBS is Campylobacter jejuni infection.(11) Cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus (HIV), and Zika virus have also been associated with GBS.(11-13)
Cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus (HIV), and Zika virus have also been associated with GBS.(11-13)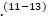 A small number of patients develop GBS after other events such as immunization, surgery, trauma, and bone-marrow transplantation.(11)(13)
A small number of patients develop GBS after other events such as immunization, surgery, trauma, and bone-marrow transplantation.(11)(13)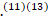
According to studies from the United States and Europe, reflecting primarily patients with acute inflammatory demyelinating polyneuropathy (AIDP), GBS is associated with weakness usually starting in the legs, but it can begin in the arms or facial muscles in about 10% of patients.(11)(14)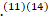 Furthermore, Severe respiratory muscle weakness necessitating ventilatory support develops in 10-30%, Facial nerve palsies in ≥50%, and oropharyngeal weakness in 50% of cases. At presentation, approximately 90 per-cent of patients will have decreased or absent reflexes in affected arms or legs and in all patients with disease progression.(11)(14-16)
Furthermore, Severe respiratory muscle weakness necessitating ventilatory support develops in 10-30%, Facial nerve palsies in ≥50%, and oropharyngeal weakness in 50% of cases. At presentation, approximately 90 per-cent of patients will have decreased or absent reflexes in affected arms or legs and in all patients with disease progression.(11)(14-16)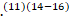
Looking at the disease course in our patient, he presented firstly with mild respiratory symptoms include fever, cough along with loss of appetite. During his hospital stay, the patient was doing well with no newly developed symptoms; therefore, he was discharged from the hospital. About two weeks after discharge and six weeks after confirmed diagnosis with COVID-19, the patient presented with severe dysarthria along with bilateral lower limb weakness (paraplegia) that progressively ascended over two weeks to involve all limbs (quadriplegia). There was some delay in the diagnosis of GBS, as the initial management was based on the assumption that the brain MRI findings can explain the paraplegia. The brain abscesses and surrounding oedema were involving the motor area along the pre-central gyrus of the frontal lobes bilaterally especially the lower limbs according to the motor homunculus topographic representation of the body parts (Figure. 1). However, with the progression of his condition to quadriplegia, and with normal MRI cervical spine findings, the suspicion of GBS post COVID-19 infection was raised. EMG was performed two times and confirmed the diagnosis, as there was a significant proximal conduction block (Table 1&2) (Tables 3&4).
Cerebral abscess as neurological complications post COVID-19 infection has not been reported yet. In our patient, he is known to have CLL; therefore being immune-compromised could have increased the risk of developing fungal brain abscesses. How much did brain abscesses contribute to the development of GBS remains to be determined. In addition, since SARS-CoV-2 entry into brain tissue via spread and dissemination from the cribriform plate is one of the proposed mechanisms targeting the CNS,(19) cerebral abscess might be one of the neurological complications occurring in COVID-19 patients especially those who are mainly immune-compromised as ethmoidal and frontal sinusitis is one of the established contiguous routes for frontal brain abscess. This might explain the occurrence of frontal brain abscess in association with GBS and COVID-19 infection in our patient. In addition, re-infection with COVID-19 is controversial and not been proven yet, however, in our patient he got re-infected again as confirmed by the nasopharyngeal swab, with about 3 months gab from the initial infection.
cerebral abscess might be one of the neurological complications occurring in COVID-19 patients especially those who are mainly immune-compromised as ethmoidal and frontal sinusitis is one of the established contiguous routes for frontal brain abscess. This might explain the occurrence of frontal brain abscess in association with GBS and COVID-19 infection in our patient. In addition, re-infection with COVID-19 is controversial and not been proven yet, however, in our patient he got re-infected again as confirmed by the nasopharyngeal swab, with about 3 months gab from the initial infection.
Conclusion
To date, nine adults and one paediatric case,(1)(5-10)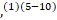 described the association between GBS and COVID-19 infection in the literature. Despite the number of case reports of GBS associated with COVID-19 infection, the prevalence remains uncertain.(17)(18)
described the association between GBS and COVID-19 infection in the literature. Despite the number of case reports of GBS associated with COVID-19 infection, the prevalence remains uncertain.(17)(18) However, GBS should be recognized as one of the differential diagnosis in patients with COVID-19 presenting with bilateral limbs weakness for early detection and treatment. In our case, despite an established pathology (brain abscess), GBS was an additional factor that explains the patient limbs weakness due to association between SARS-CoV-2 and GBS. Furthermore, cerebral abscess might be one of the neurological complications linked to COVID-19 infection. Re-infection with COVID-19 is possible and should be taking into consideration for healthcare precautions.
However, GBS should be recognized as one of the differential diagnosis in patients with COVID-19 presenting with bilateral limbs weakness for early detection and treatment. In our case, despite an established pathology (brain abscess), GBS was an additional factor that explains the patient limbs weakness due to association between SARS-CoV-2 and GBS. Furthermore, cerebral abscess might be one of the neurological complications linked to COVID-19 infection. Re-infection with COVID-19 is possible and should be taking into consideration for healthcare precautions.
Conflict of interest statement:
We declare that this paper content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical consideration:
An informed consent about the case report was taken from the sons of the patient.
Abbreviations:
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2
GCS: Glasgow coma score
GBS: Guillain-Barré syndrome
MRI: Magnetic Resonance imaging
CT: Computerized Tomography
CXR: Chest-X-Ray
EMG: Electromyography