Neurosurgery and Neurology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2025
ISSN No: 2836-2829 | Journal DOI: 10.61148/2836-2829/NNR
Mohammed H Habib
Department of Cardiology and Cardiac Catheterization, Al-Shifa Hospital, Gaza, Palestine
*Correspondence author: Mohammed H Habib, Department Of Cardiology And Cardiac Catheterization, Al-Shifa Hospital, Gaza, Palestin.
Received date: May 29, 2021
Accepted date: June 09, 2021
published date: June 12, 2021
Citation: Mohammed H Habib. “Acute Ischemic Stroke After Recent Myocradial Infarction”. J Neurosurgery and Neurology Research, 2(3); DOI: http;//doi.org/06.2021/1.1019.
Copyright: © 2021 Mohammed H Habib. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Acute ischemic stroke and coronary artery disease are the major causes of death in Palestine and in the world. The prevalence of coronary artery disease has been reported in one fifth of stroke patients. Although high risk of acute ischemic stroke after recent myocardial infarction has been reported in several clinical or observational studies. So that acute or recent problem in the heart could result in an acute infarction of the brain.
Introduction
The incidence of acute ischemic stroke (AIS) after recent myocardial infarction (MI) during the hospital stay ranges from 0.7% to 2.2%. (1-3) AIS occurred more frequently in the first days after Acute myocardial infarction (AMI), but incidence progressively decreased over time. (3-5) Brandi Witt et al, suggested that during hospitalization for MI 11.1 the AIS occurred per 1000 MI compared with 12.2 at one month and 21.4 at one year. The most positive predictors of ischemic stroke after MI included: older age, hypertension, diabetes, history of previous stroke, history of anterior location MI, previous MI, atrial fibrillation and heart failure (6).
Acute ischemic stroke after recent myocardial infarction
Definition: Acute ischemic stroke < 4.5 hours (if patients awake with stroke symptoms or have unclear time of onset > 4.5 hours from last known well, MRI with diffusion-positive FLAIR negative lesions). in patients with history of recent myocardial infarction in the previous 3 months but more than 12 hours
Diagnosis: AIS (a sudden onset of focal neurological deficit caused by an cerebral vascular narrowing cause) and recent history of MI (acute elevation cardiac enzyme plus ischemic electrocardiogram changes and/or symptoms ) in the previous 3 months but not in first 12 hours from MI.
Pathophysiology (table 1):
left ventricular mural thrombus (LVMT) due to impaired left ventricle ejection fraction (EF) <35% and regional wall motion abnormalities such as dyskinesia or akinesia and septi-apical wall role the most important risk factor. The LVMT is most likely to occur by 2 weeks after an AMI in 0.6–3.7% of patients (7) . new pharmacological therapy with primary PCI procedures and dual antiplatelet agent, might have contributed to the decreases of LVMT formation after myocardial infarction (8). Increased coagulation activity during AMI, , can potentially lead to increased thrombosis and subsequent thromboembolic events including stroke.
The circulatory inflammatory cytokines may be initiated a cascade of events in the cerebral circulation. this phenomenon may contribute to plaque rupture and subsequent thrombus formation in the cerebral circulation (9).
Revascularization with early PCI has become the standard of care for patients with acute myocardial infarction and coronary artery bypass graft surgery (CABG) were associated with increased stroke risk. Similarly, analysis of the OASIS (10) registry found that patients with higher rates of invasive cardiac procedures (CABG and PCI) suffered from increased risk of ischemic stroke at 6 months (p = 0.004).
Atrial fibrillation (AF) and atrial flutter after myocardial infarction increased risk of ischemic stroke and occurs in up to 20% of patients and can cause increased in-hospital and long-term mortality (11)

Table 1: Causes of acute ischemic stroke after myocardial infarction
PCI: percutaneous coronary intervention, CABG: coronary artery bypass graft surgery
Treatment:
According to the 2018 guideline of scientific statement from the American Heart
Association/American Stroke Association (AHA/ASA), (12)
For patients presenting with AIS and a history of recent MI in the past 3 months, treating the ischemic stroke with IV alteplase is reasonable if the recent MI was non-STEMI. (Class IIa)
For patients presenting with AIS and a history of recent MI in the past 3 months, treating the ischemic stroke with IV alteplase is reasonable if the recent MI was a STEMI involving the right or inferior myocardium. (Class IIa)
For patients presenting with AIS and a history of recent MI in the past 3 months, treating the ischemic stroke with IV alteplase may reasonable if the recent MI was a STEMI involving the left anterior myocardium. (Class IIb)
The main concerns about giving rt-PA to patients with AIS and history of recent MI are (Beyond the bleeding):
Thrombolysis-induced myocardial hemorrhage predisposing to myocardial wall rupture
Possible ventricular thrombus that could be embolize because of thrombolysis.
post-myocardial infarction pericarditis that may become hemopericardium
The safety of IV rt-PA for acute ischemic stroke (AIS) treatment after recent myocardial infarction (MI) is still controversial. In recent Retrospective review article of 102 AIS patients admitted for AIS with history of recent MI in the previous 3 months. Patients according to treated with standard IV rt-PA dose for AIS were divided into 2 groups: treated or not treated. Four patients with STEMI patients in the week preceding ischemic stroke (8.5%) and IV rt-PA treated died from confirmed cardiac rupture/ tamponade. This complication occurred in 1 (1.8%) patient in the nontreated group (P=0.178), and no non-STEMI patients receiving IV rt-PA had cardiac complications [13].The new recommendation according to 2021 guidelines of European Stroke Organization (ESO) on intravenous thrombolysis for acute ischemic stroke suggested that [14]:
Contraindication of rt-PA For patients with acute ischemic stroke of < 4.5 h duration and with history of subacute (> 6 h) ST elevation myocardial infarction during the last seven days.
Insufficient evidence to make a recommendation for patients with acute ischemic stroke of < 4.5 h duration and with history of ST-elevation myocardial infarction of more than a week to three months.
IV rt-PA for patients with acute ischemic stroke of < 4.5 h duration and with a history of non-ST-elevation myocardial infarction during the last three months.
The recent retrospective trial among 40 396 AIS patients with age ≥ 65 years, the patients treated with rt-PA were 241 patients (0.6%) had recent MI in the past 3 months, of which 19.5% (41 patients) were ST-segment–elevation myocardial infarction. Patients with recent MI had more severe stroke than those without. Among older patients receiving rt-PA for AIS, a recent history of MI in the past 3 months was associated with higher in-hospital mortality compared with no history of MI in ischemic stroke patients treated with rt-PA. This association was more prominent in patients with STEMI than those with NSTEMI. This association was not significant, if the time frame from the onset of MI to the indexed AIS was > 3 months [15].
Despite the increasing risk of mortality, further studies are necessary to determine whether the benefit of rt-PA outweighs its risk among AIS patients with a recent history of MI in last 3 months.
Thus, we recommended treatment (figure 1):
Intravenous rt-PA for patients with acute ischemic stroke of < 4.5 h duration and with a history of non-STEMI during the last three months.
No intravenous rt-PA for patients with acute ischemic stroke of < 4.5 h duration and with history of ST-elevation myocardial infarction of less than one week but more than 12 hours. Mechanical thrombectomy may be a therapeutic alternative in this patient with large vessel occlusion.
For patients with acute ischemic stroke of < 4.5 h duration and with history of ST-elevation myocardial infarction of more than one week to three months, there is insufficient evidence to make a recommendation, IV alteplase is reasonable if history of STEMI involving the right or inferior myocardium. But not recommended in patients with history of anterior MI. Mechanical thrombectomy may be a therapeutic alternative in this patients with large vessel occlusion.
Anticoagulation with novel oral anticoagulation (such as Rivaroxaban) and clopidogrel is recommended in patients with AIS related to cardioembolic causes ( left ventricle thrombus and/or atrial fibrillation) and must be at least 3 months then aspirin lifelong for left ventricle thrombus and 3 months rivaroxaban and clopidogrel then rivaroxaban lifelong for atrial fibrillation (16).
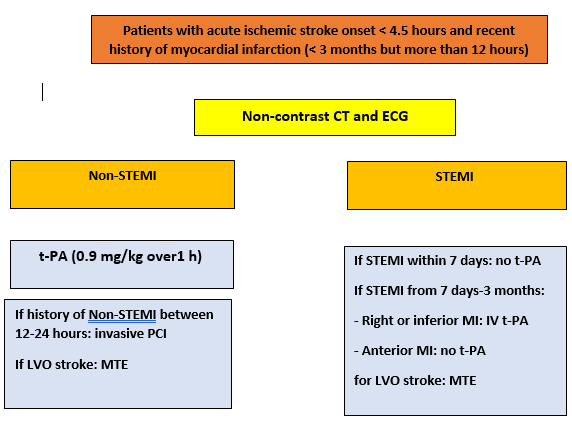
Figure 1: Treatment of ischemic stroke onset and recent history of myocardial infarction
LVO: large vessel occlusion, MTE: mechanical thrombectomy, non-STEMI: non ST elevation myocardial infarction, STEMI: ST elevation myocardial infarction, ECG: Electrocardiogram
Recommendations of antithrombotic therapy
The cardioembolic causes treatment must be included novel oral anticoagulation (NOAC) and prefer (Rivaroxaban) or oral anticoagulation OAC (warfarin) and dual or single antiplatelet according to 2020 non-ST elevation acute coronary syndrome guideline of European Society of Cardiology (29) and to prevention of bleeding in patients with Atrial Fibrillation undergoing PCI trial (17). In single antiplatelet with (novel) oral anticoagulation (N) OAC preference for a clopidogrel over aspirin and prefer NOAC over OAC for the default strategy and in all other scenarios if no contraindications ( Prosthetic valve or moderate to severe mitral stenosis). Algorithm for antithrombotic therapy and dosage listed of the following (figure 2) :
AF: (Clopidogrel (75 mg)+ (N) OAC (Rivaroxaban 15 mg OD (GFR <60: 10 mg) or warfarin: INR 2-3 and TTR > 70%)
LVMT: first 3 months: (Clopidogrel (75 mg)+ OAC (Rivaroxaban 15 mg OD (GFR <60: 10 mg) or warfarin: INR 2-3 and TTR > 70%). after 3 months: Aspirin (75-100 mg) + Clopidogrel (75 mg).
If patient high risk of bleeding the duration of dual therapy can be reduce from one year to 6 months.
AF: Rivaroxaban or warfarin (Rivaroxaban 20 mg OD (GFR <60: 15 mg) or warfarin: INR 2-3 and TTR > 70%), LVT: aspirin 100 mg tab once daily. If patient high risk of bleeding start only single antiplatelet or (N)OAC at 6 months for life long

Figure 2: Algorithm for antithrombotic therapy
(N)OAC (novel) oral anticoagulation (N), SAPT single antiplatelet therapy, DAPT dual antiplatelet therapy
Initiation of anticoagulation (N)OAC after ischemic stroke:
Patients with a small stroke with National Institutes of health scale score (NIHSS) < 8 may benefit from early initiation of anticoagulation. But in large ischemic stroke with NIHSS > 8 initiate of anticoagulation in AF patients between 1 and 12 days after an ischemic stroke, depending on stroke severity.
In patient with NIHSS 8-15 anticoagulation initiate 6 days after an ischemic stroke, and if NIHSS > 16 initiate of anticoagulation must be >12 days after ischemic stroke. We suggest repeat brain imaging to determine the optimal initiation of anticoagulation in patients with a large stroke at risk for hemorrhagic transformation. NOACs seem to convey slightly better outcomes, mainly driven by fewer intracranial hemorrhages and hemorrhagic stroke (figure 3)
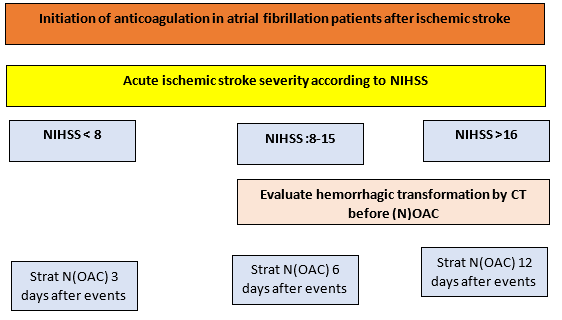
Figure 3: Initiation of anticoagulation
NIHSS: National Institutes of health scale score, CT: Computed Tomography (N)OAC: (New) oral anticoagulation
Recommendations of Lipid-lowering drugs (18-20)
High intensity statins are recommended in all MI and/or AIS patients. The aim of treatment is to reduce LDL-C by > 50% from baseline and to achieve LDL-C <1.4 mmol/L (<55 mg/dL).
If the target LDL-C is not achieved after 4-6 weeks with the maximally tolerated high intensity statin dose, we recommended combination of statin with ezetimibe.
If the target LDL-C is not achieved after 4-6 weeks despite maximally tolerated high intensity statin therapy and ezetimibe, we recommended the addition of a PCSK9 inhibitor to statin and ezetimibe.
Recommendations of (antihypertensive/anti-ischemic/anti failure drugs)
Angiotensin-converting enzyme (ACE) inhibitors or Angiotensin receptor blocker (ARBs) are recommended in patients with heart failure with reduced LVEF (<40%), diabetes, hypertensive or Chronic kidney diseased unless contraindicated (e.g. severe renal impairment, hyperkalaemia, etc.) (21)
Beta-blockers are recommended in patients with prior MI, long-term oral treatment with a beta-blocker should be considered in order to reduce all-cause and cardiovascular mortality and morbidity and in patients with systolic LV dysfunction or heart failure with reduced LVEF (<40%). (22-25)
Mineralocorticoid receptor antagonist (MRAs) are recommended in patients with heart failure with reduced LVEF <40% in to reduce all-cause and cardiovascular mortality and morbidity. (26-28)
Recommendations of Proton pump inhibitors: (29)
In patients with dual antiplatelet and higher risk of gastrointestinal bleeding:
History of gastrointestinal bleeding or ulcer,
Corticosteroid use,
Oral anti-coagulant therapy,
Use of non-steroidal anti-inflammatory drugs, or two or more of
Old age more than 65 years.
Gastro-esophageal reflux disease.
History of Helicobacter pylori infection.
Dyspepsia.
Conclusion
For patients with acute ischemic stroke of < 4.5 h duration and with a history of recent non-ST-elevation myocardial infarction during the last three months, we suggest intravenous thrombolysis with alteplase, and Mechanical thrombectomy may be a therapeutic alternative in patients with large vessel occlusion and recent STEMI.
A Conflict of interest: No conflit of interest