
Priya Senthil
Department of Reproductive Medicine, Saveetha Institute of Medical Sciences Thandalam, Chennai
*Corresponding author: Priya Senthil Department of Reproductive Medicine, Saveetha Institute of Medical Sciences Thandalam, Chennai
*Corresponding author: Priya Senthil Department of Reproductive Medicine, Saveetha Institute of Medical Sciences Thandalam, Chennai
Received date: January 06, 2021
Accepted date: January 19, 202
Published date: January 22, 202
Citation: Senthil P., (202) Comparison of Oocyte Maturation in Recombinant Human Chorionic Gonadotrophin Trigger and Triptorelin Acetate Trigger; A Randomised Prospective Study. Women’s Health Care And Analysis, 2(1); DOI: http;//doi.org/03.2020/1.1005
Copyright: © 202 Priya Senthil, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
LH like exposure in the mid cycle for inducing the oocyte maturation , is the very crucial step in the success of ICSI treatment . Eliciting an endogenous LH surge by GnRH-agonist for the induction of final oocyte maturation may be more physiological compared with the administration of HCG. Since it would cause FSH surge also along with LH surge , as in natural cycle . However, the efficacy of this intervention in patients treated for ICSI with GnRH antagonists and triggered with Triptorelin acetate and recombinant Human
Chorionic Gonadotrophin (HCG) triggers remains to be assessed more. Sub fertile patients planned for ICSI, meeting the requirement of inclusion criteria , were started with recombinant FSH from Day 2 or 3 of menstrual cycle . GnRH Antagonist were started from Day 5 of stimulation. FSH dose adjusted according to the individual response. Trigger was planned when 2 or 3 follicles reach 16-22 mm. For triggering, 100 patients were randomised to receive Recombinant HCG trigger and Triptorelin acetate trigger. Oocyte retrieval was done 36 hours after Recombinant hCG Trigger and 35 hours after Triptorelin acetate trigger. The Oocyte maturity rate was assessed by the number of metaphase II oocytes retrieved.
GnRH Agonist Trigger; GnRH Antagonist cycle; Metaphase 2 oocytes; Oocyte maturation rate; Recombinant hCG Trigger; Triptorelin; Trigger
Introduction
Advances in assisted reproduction has provided hope to subfertile couples. Infertility affects one in six couples and is recognized by the World Health Organization (WHO) as the fifth most serious global disability (WHO). However, former director of the WHO Human Reproductive Program explained the rationale for this: “If public health policies encourage couples to delay and plan pregnancies, then it is equally important that they are assisted in their attempts to conceive in the more limited time available”(Mahmoud Fathalla,)
Controlled ovarian hyper stimulation (COH)is a supra physiological process that simulates the
physiological process occurring during the normal menstrual cycle, namely follicular
development, oocyte maturation, ovulation. Gonadotrophins -Follicular stimulating hormone(FSH) and/
or Leutinising hormone(LH) are given from day 2 or day 3 of cycle, to induce the growth of multiple
ovarian follicles. As follicles grow, premature LH surge can occur which can cause ovulation. It is
prevented either through the use of a GnRH (Gonadotropin releasing Hormone) antagonist (Castillo JC et al 2014, Humaidan et al 2011) or continuous administration of a GnRH agonist (GnRHa) (Depalo R et al 2013).
Once follicles reach the optimal size, LH exposure is provided through Human chorionic gonadotropin (HCG) or GnRH agonist, to simulate the mid-cycle LH surge, which induces the process of oocyte maturation and subsequent ovulation (Shoham Z et al 1995). Oocyte retrieval is thus precisely timed following provision of LH exposure to retrieve oocytes following oocyte maturation, but prior to the occurrence of ovulation. LH exposure initiates the resumption of meiosis and the maturation of the oocyte from the immature “metaphase 1” stage to the mature “metaphase II” stage of development ( Voronina E et al 2003).
Thus, the LH-like exposure required to initiate the process of oocyte maturation is a crucial step in the success of ovarian stimulation protocols enabling the efficacious retrieval of mature oocytes, In current ovarian stimulation protocols, LH-like exposure is provided through the use of human chorionic gonadotropin (hCG) and/or GnRHa, which are referred as the “trigger” of oocyte maturation (Castillo JC et al 2014).
Controlled ovarian hyper stimulation (COH) and induction of final oocyte maturation are the leading limiting steps in artificial reproductive techniques. Approximately 20% of collected oocytes obtained from COH may have nuclear or cytoplasmic immaturity; either at the MI or GV (I. Halvaei 2012).Ultimately oocyte competence is dependent on the completion of two major interdependent largely microscopic molecular processes, one nuclear and the other cytoplasmic. While the extrusion of the 1st PB indicates the completion of nuclear maturation it may bear no reflection of the quality of the genome of the oocyte. Although cytoplasmic maturation is just as critical to the competence of an oocyte there are no macroscopic markers indicating its completion (H.Balakier 2004, Grendahl 2008) Oocyte maturation has been defined as the period taken by an oocyte to progress from the first to the second meiotic metaphase, which involves coordinated, but asynchronous nuclear and cytoplasmic maturational modifications Grendahl 2008, Sirard 2011)
ART is a complex and costly technique with stringent indications. Providing infertile couples with accurate information about their chances of pregnancy is a priority in all ART programs. Although many factors influence the success of ART, there is a general consensus that the key role is played by ovarian response to stimulation. The more the adequately matured oocytes collected, the more the good embryos develop for transfer. Nonetheless, there is still a portion of premature and hypermature oocytes collected.
In this study, we aim to discuss the predominant hormonal stimuli used to induce final oocyte
maturation, with a particular focus on the endocrine requirements for efficacy that is retrieval of
mature oocytes following the use of recombinant hCG vs. Triptorelin acetate (GnRH agonist).
Aim And Objective
Aim : To compare Oocyte Maturation In Recombinant Human Chorionic Gonadotrophin Trigger And Triptorelin Acetate Trigger in ICSI Freeze all ART cycles
PRIMARY OBJECTIVE
To compare the oocyte maturation in patients undergoing controlled ovarian hyper stimulation in GnRH antagonist cycle , ovulation induced with Triptorelin acetate trigger and Recombinant HCG trigger . Oocyte maturity was defined as the ratio of metaphase II (MII) oocytes to the total number of oocytes collected.
SECONDARY OBJECTIVE
To compare the incidence of ovarian hyper stimulation syndrome in both trigger groups
Materials and Method
Study Population
Total of 100 patients undergoing controlled hyper stimulation in GnRH antagonist cycle, 50 each in Triptorelin acetate trigger group and Recombinant HCG trigger group indicated for ICSI ARC fertility centers (affiliated to Saveetha University) meeting inclusion criteria and willing to participate in the study.
Inclusion Criteria
Subfertile patients aged 21-35 years with Body mass index (BMI) between 18-30 , Antral follicular count between 8-24 , Anti mullerian hormone level 1.6-4 ng/dL and serum estradiol value on the day of trigger between 3000-5000 pg/mL.
Exclusion Criteria
Subfertile patients aged more than 35 years, Body mass index less than 18 and more than 30, Antral follicular count less than 8 and more than 24, Anti mullerian hormone level less than 1.5 ng/ dL and more than 4 ng/ dL and serum estradiol level on the day of trigger less than 3000 pg/mL and more than 5000 pg/mL. And also previous history of chemotherapy like methotrexate, history of oopherectomy , history of previous ICSI failure.
Monitoring
Controlled ovarian hyper stimulation with recombinant FSH was started from day 2 or day 3 of cycle after confirming the pituitary down regulation, ie. serum estradiol level less than 50 pg/mL,
Serum progesterone level less than 0.9 ng/mL, serum Leutinising hormone level less than 5 IU/L Endometrial thickness less than 5 mm, No antral follicle larger than 10 mm in each ovary. Starting dose of Recombinant FSH was based on patient’s age, body mass index BMI , antral follicular count and serum anti mullerian hormone level. Inj Cetrorelix 0.25 mg was started subcutaneously from Day 6 of cycle . Follicular growth was assessed by serial transvaginal and serum E2, LH and P4 levels, and Recombinant FSH doses were adjusted accordingly. Ultrasound monitoring of follicular growth was done on day 6, day 8, day10 and then daily till trigger .Serum Estrogen was measured on day 6, day 8 and day10 and before trigger. Serum LH was measured on day 6, day8 and day10. And Inj cetrorelix was continued till the day of the trigger. Trigger wass planned when 2 or more follicles reach 18-22 mm size .For ovulation trigger, the patients with serum estradiol level between 3001-5000 pg/dL were randomly divided into two groups: one group received recombinant HCG 250 mg subcutaneously and the other group received Triptorelin acetate 0.2 mg, a GnRH Agonist subcutaneously as ovulation Trigger . Both groups were randomised by a standard randomisation software by the computer. And serum progesterone level was measured 24 hours after the trigger.
Oocyte Retrieval was done 36 hours of Recombinant HCG Trigger and 35 hours after Triptorelin trigger. Oocyte cumulus complexes were cultured in a medium containing hyaluronidase for 1-2 hours. Oocytes were separated from the cumulus complex and the maturity of the oocytes was assessed and graded as “Metaphase 2” oocytes when the Oocyte has visible polar body and “Metaphase 1” when there was no visible Polar body and no germinal vesicle in the ooplasm and “Germinal vesicle” when the germinal vesicle was seen.
Post OCR follow up
For patients triggered with recombinant HCG, when less than 15 oocytes were retrieved, GnRH antagonist Inj Cetrorelix 0.25 mg s.c administered for 3 days . T. Cabergoline 0.5 mg once a day orally for 3 days , Calcium gluconate administered once a day intravenously for 3 days . And when 15 or more oocytes retrieved , GnRH antagonist 1 gm s.c administered for 3 days , T. Cabergoline 0.5 mg once a day orally for 3 days , Calcium gluconate administered once a day intravenously for 3 days .
For patients triggered with Triptorelin acetate, GnRH antagonist was not given post OCR. Neither Inj Calcium gluconate was given . Tablet Cabergoline 0.5 mg once day was given orally for 3 days.
All patients were advised to have protein rich diet , walking , restricted fluid intake 1-1.5 litres for next 4-7 days . In case of symptoms such as abdominal distension, pain, nausea, vomiting, diarrhea, or any difficulty in breathing, patients were advised to report to the clinic at any point of time (24 X 7) within 2 weeks of trigger.
The diagnosis of OHSS was based on the criteria described by Navot et al[8]. An assessment for signs and symptoms of OHSS was performed on day 1, day 4 and 7 following oocyte retrieval which included a history and physical examination by a clinician. Transvaginal sonography was done in patients, to assess the ovarian size, free fluid in pouch of Douglas, paracolic gutters, Morrison's pouch, pleura,. Blood sampling for E2 and to detect hemoconcentration ,defined as a hematocrit of 45%[8] was performed.
Review of Literature
The Menstrual Cycle- Natural versus stimulated ;
During the early follicular phase of the natural cycle, serum estradiol and progesterone levels are low and inhibin B is secreted by small antral follicles (43 Welt CK 2001). Thus, the early follicular phase is characterized by both reduced negative feedback from low sex steroid levels and decreased negative feedback on FSH secretion by inhibin B levels (44 Keogh EJ 1976,45 Luisi S 2005), overall resulting in an ~30% increase in serum FSH (46. Hall JE et all 1992, 47.Welt CK 1999, 48.Burger HG 1998,). This modest rise in FSH stimulates folliculogenesis and aromatase action to increase estradiol production by ovarian granulosa cells (49. Brown JB 1978).
The FSH intercycle rise is the result of the reduced activity of the negative feedback mechanism due to the decrease in estradiol (E2), inhibin A and progesterone (P4) concentrations in late luteal phase. The FSH increase is terminated by the rising E2 and inhibin B levels produced by the dominant follicle.
However, multi follicular development during controlled ovarian hyperstimulation may alter the hormonal milieu from the natural cycle. As estradiol levels continue to rise, there is a critical switch from negative to positive feedback on GnRH secretion (51.Laws SC et al 1990), which increases LH synthesis and lowers the GnRH concentration required for LH production (52.Ramey JW et al 1987). And although estradiol is key in initiation of the mid-cycle LH surge (55,56), levels of progesterone during the follicular phase are also influential. Administration of progesterone can advance the timing of the LH surge (55. Liu JH 1983, 57. March CM 1979). coadministration of progesterone with estradiol results in an LH surge of greater duration and amplitude than by estradiol alone (58. Messinis IE 1990, 60. Fowler PA 2001). Gonadotropin surge-attenuating factor (GnSAF) a molecule produced by ovarian follicles (59) reduces pituitary sensitivity to GnRH (60. Fowler PA et al 2001) and attenuate the amplitude of the LH surge (61. Messinis IE et al 2006). Differences in GnSAF contribute to the differential sensitivity to GnRH antagonism observed between cycles with mono follicular and multi follicular growth, where by hyper secretion of GnSAF in cycles with multi follicular development may reduce the degree of GnRH antagonism required to prevent a premature LH surge (61 Messinis IE et al 2006). Role of Kisspeptin for mediating LH surge after estradiol plateau is also being studied. Kisspeptin downregulates the FSH receptors at the same time promoting final maturation and ovulation.
Oocyte Maturation
Final oocyte maturation is the process by which the oocyte resumes meiosis to transition from the metaphase I to the metaphase II stage of development, at which stage it attains competence for fertilization by a spermatozoon (8) and also the capacity to support embryo development to the blastocyst stage and to live birth (62. Conti M et al 2018 ). It is initiated by LH-like exposure that induces a fall in intraoocyte cAMP and is commonly assessed by the production of a polar body to signify a mature/ metaphase II oocyte (63. Adams TE et al 1986 ). Though the process of meiosis is initiated during embryogenesis , it is halted after prophase with the nucleus contained within an intact envelope and possessing condensed chromatin (64. Jaffe LA et al 2017). At this stage, the oocyte is surrounded by precursors of follicular somatic cells(granulosa cells) in a single squamous layer, forming the primordial follicle. Oocyte meiotic development remains arrested at this stage until antrum formation (65. Eppig JJ et al 2001). Pituitary release of gonadotropins following acquisition of reproductive maturity at puberty stimulates follicular and oocyte growth, resulting in the formation of primary and secondary follicles. Thus, whereas primordial follicle growth is a gonadotropin-independent continuous process (66.McGee EA et all 2000), secondary recruitment is gonadotropin-dependent. Crosstalk with cumulus cells play an important role in oocyte maturation, providing the oocyte with metabolic support and regulatory cues (67.Coticchio G et al 2015).
Nuclear maturation
Although nuclear and cytoplasmic maturation are linked processes, cytoplasmic maturation can occur independently of full nuclear maturation (67. Coticchio G wt al 2004) [Fig. (69. Mao L et al 2014 ) for exposition of nuclear and cytoplasmic oocyte maturation. During the initial growth phase of the oocyte, nuclear chromatin decondenses and is transcriptionally active. As folliculogenesis progresses, the oocyte acquires meiotic competence, as identified by the condensing and nuclear association of chromatin, and the formation of microtubule organizing centers, necessary for spindle formation (70. De La Fuente R 2006,71. Schuh M et al 2007). Yet, although the oocyte now possesses the ability to progress through meiosis, this only occurs if the oocyte is removed from the follicle, with follicular signals ensuring that oocyte development is arrested at prophase I (62. Conti M et al 2018). This allows the oocyte to undergo further differentiation between the late antral and periovulatory follicular stages, affording the oocyte developmental competence to sustain embryo development (62. Conti M et al 2018).
Developmental competence requires a series of nuclear and cytoplasmic cellular events that take place alongside meiotic stages to enable fertilization, DNA replication, and zygoteploidy. The resumption of meiosis is signaled by germinal vesicle breakdown (GVBD). Oocytes then progress through metaphase I in which paired homologous chromosomes align in the middle of the forming meiotic spindle. Nuclear chromosomes then separate, with half the genetic material being extruded in the first polar body, resulting in the formation of a mature, haploid, metaphase II oocyte, with competence for fertilization (64. Jaffe LA et al 2017). Normal meiotic spindle morphology in meta-phase II oocytes assessed by polarized light microscopy and poloscopy guided ICSI was more likely to result in an euploid embryo (72. Tilia L et al 2016). Ameta-analysis of 10 studies determined that when the meiotic spindle was present, fertilization rates were significantly higher (P< 0.0001), as were cleavage rates (P<0.0001) and the proportion of top-quality cleavage embryos (P = 0.003) (73.Petersen CG et al 2009). The interval to GVBD is difficult to assess but is estimated to occur at a median of 6.5 hours and the interval between LH receptor activation and the first stage of meiosis is thought to be about 18hours (74.Seibel MM et al 1982). In humans, spindle assembly typically occurs about 10hours following GVBD, and about 14 to 20 hours are required between GVBD and polar body extrusion (62. Conti M et al 2018). The total duration of nuclear maturation including the time to GVBD has been estimated to be about 20 to 22 hours (75.Escrich L et al 2012). The oocyte then remains at metaphase II until fertilization (68.Coticchio G et al 2004).
Cytoplasmic maturation
Cytoplasmic maturation prepares the oocyte for nuclear maturation with specific chromatin configurations indicating the likelihood of the oocyte to resume meiosis (67).Thus, nuclear maturation mainly comprises chromosomal segregation, whereas cytoplasmic maturation involves organelle redistribution, changes in cytoskeletal dynamics, Golgi apparatus, calcium releasing activity, storage of mRNAs, proteins and transcription factors (69. Mao L et al 2014). Cytoskeletal changes in microtubules, actin filaments, and chromatin create cell asymmetry and enable polar body extrusion with minimal loss of cytoplasm (69. Mao L et al 2014). Al though nuclear maturation is apparent by the presence of the extruded first polar body, it is more challenging to assess cytoplasmic maturity in clinical practice (76. Pereira N et al 2016). Whereas the oocyte only provides half of the genetic material, it provides nearly all membranous and cytoplasmic determinants required for embryogenesis. At metaphase II, the endoplasmic reticulum is redistributed from a fine network into clusters throughout the oocyte, and more functionally competent mitochondria are found beneath the oolemma.
Maintenance of meiotic arrest
The prophase arrest is in part maintained by the oocyte itself (64 Jaffe LA et al 2017). Cyclin-dependent kinase 1 (CDK1) is a protein expressed by the oocyte that triggers chromosomal condensation and nuclear laminar breakdown, and thus is necessary for the progression from prophase to metaphase I. As the oocyte increases in size, so too does expression of CDK1; however, despite this, the oocyte remains arrested in prophase until it is removed from the follicle. This suggests that inhibitory factors from granulosa cells play an important role in preventing the resumption of meiosis (77). In follicle-enclosed oocytes, LH results in a decrease in cAMP (78. Sela- Abramovich S et al 2016) and cyclic guanosine monophosphate (cGMP) to mediate the resumption of meiosis (79. Norris RP et al 2009 ,80. Vaccari S et al 2009). cAMP maintains CDK1 kinase in its inactive form through the action of protein kinase A. Thus, a fall in cAMP allows the formation of active forms of CDK1 kinase to initiate a cascade of events culminating in the resumption of meiosis (64.Jaffe LA et al 2017). cGMP is produced by granulosa cells and subsequently diffuses into the oocyte via gap junctions (64. Jaffe LA et al2017), where it competitively inhibits the action of cAMP phosphodiesterase (80. Vaccari S et al 2009). By preventing cAMP hydrolysis, cGMP therefore helps to maintain meiotic arrest. In summary, human oocytes are arrested at meiotic prophase I until the mid-cycle LH surge signals a series of intracellular changes, including changes in oocyte and granulosa cell cAMP/cGMP levels that result in the resumption of meiosis, and the development of a metaphase II oocyte.
Endocrine Requirements for Oocyte Maturation and Ovulation
There is a threshold value for LH to initiate the process of oocyte maturation/ovulation, and once this level is exceeded, the duration of exposure is more critical for inducing ovulation and supporting functional corpora lutea Natural ovulatory LH surge consists traditionally of three phases: abrupt onset (14 hours), LH peak/plateau (14 hours), and gradual descent to baseline (20 hours), lasting a mean duration of 48 hours (Figure ) (39). In contrast, the surge after GnRHa occurs in two phases: rapid ascent and moderate descent, lasting 24–36 hours (3). And after HCG there is only LH surge no FSH surge .
An early study by Itskovitz et al. (3) showed that GnRHa causes LH to rise over four hours and FSH to rise over 12 hours, with significant elevation lasting 24 hours before a return of LH to baseline levels (3,20). The relatively short duration of the LH surge is capable of inducing oocyte maturation and ovulation but may result in defective formation of the corpus luteum (40).
A single bolus of GnRHa will interact with the GnRH receptors and cause the endogenous release or “flare” of gonadotropins from the anterior pituitary. The resultant surge of LH and follicle-stimulating hormone (FSH) resembles the natural mid-cycle surge of gonadotropins seen shortly before ovulation, and thus a bolus of GnRHa can “trigger” ovulation (34). While the role of the FSH surge is not completely elucidated in humans, there are animal and human cell studies suggesting that FSH plays a role in oocyte maturation and resumption of meiosis (35,36), function of the oocyte–cumulus complex and facilitation of its detachment from the follicle wall (37), and generation of LH receptors on granulosa cells (38). Thus, there may be advantages to an ovulation trigger that result in a surge of both LH and FSH. Oocyte yield was assessed by Chen et al. (167. Chen SL et al 2012 ) in 91patients who received GnRHa triptorelin at 0.2 mg 34 to 38 hours prior to oocyte retrieval. Mean serum LH at 12 hours after GnRHa was 46.6 IU/L (range, 9.7 to 151.2 IU/L), but 5.5% of patients had a serum LH less than 15 IU/L (167.Chen SL et al 2012). The oocyte yield (number of oocytes as a proportion of follicles.10 mm on day of GnRHa) was 38% and oocyte maturation rate was 77% in patients with a 12 hour serum LH less than 15 IU/L as compared with an oocyte yield of 69% to 75% and oocyte maturation rates of 79% to 84% in patients with higher LH values (167. Chen SL et al 2012). Serum LH on the day of GnRHa administration was 0.7 IU/L in patients with a 12 hour serum LH less than 15 IU/L as compared with 1.6 IU/L in remaining patients, again reinforcing the concept that a low endogenous serum LH may predict an inadequate rise in LH following GnRHa triggering (167). No significant differences were observed in oocyte maturation (167.Chen SL 2012 et al ). Similarly, in 2011,Shapiro BS et al found a modest reduction in oocyte yield (defined as proportion of oocytes from follicles more than or equal to 10 mm on day of GnRHa) and mature oocyte yield (defined as ratio of mature oocytes to the number of follicles more than or equal to 10 mm on day of GnRHa) when serum LH at 12 hours was less than 52 IU/L, but a more dramatic reduction when the serum LH was less than 12IU/L. Oocyte yield was 70% when 12 hour LH was less than 12 IU/L, 79% when 12 hour LH was less than 52 IU/L, and 86% when 12 hour LH was less than 52 IU/L (168. Shapiro BS et al 2011)
Rosen et al. in 2009 (173) noted that intra follicular FSH levels corrected for follicular size were higher in follicles that yielded an oocyte
At which follicular size to trigger
The size of follicles at the time of trigger can influence the likelihood that LH-like exposure can induce oocyte maturation. When follicles grow as a tight cohort behind the lead follicle, the lead follicle provides a reasonable representation of all of the follicles. However, when follicle sizes on day of hCG are more disparate, the lead follicle may perform less reliably as a representation of all follicles. Data on specific follicle sizes that are most likely to yield an oocyte have predominantly been generated on the day of oocyte retrieval, at which time follicles of 16 to 22 mm are thought to be most likely to yield oocytes (196. Reveli A et al 2014). Data from our own group suggest that follicles of 12 to 19 mm on the day of trigger contribute most to the number of mature oocytes retrieved (166. Abbara A et al 2018).Indeed, patients with a greater proportion of their follicles within this range had more mature oocytes retrieved (166. Abbara A et al 2018). These data also allow for a data driven estimation of trigger efficacy. Thus, we recommend that “mature oocyte yield” defined as “the proportion of mature oocytes retrieved from follicles of 12 to 19 mm” on the day of trigger is used to more accurately assess trigger efficacy. Further prospective studies are required to identify whether administering the trigger by a measure other than lead follicle size can benefit outcomes, although such a benefit may only be apparent in patients with a wide distribution of follicle size during stimulation. It should also be remembered that we are looking at a 3 D follicle in a 2D dimension, so follicular volume rather than follicular diameter may be a better parameter.
Rosen MP et al. in 2008 observed that the odds of retrieving a mature oocyte from a follicle 13 to 15 mm on the day of oocyte retrieval in size is reduced by 70% compared with follicles more than 18 mm. Dubey AK et al. in 1995(201) determined that fertilization rates were increased in oocytes from larger follicles on the day of oocyte retrieval (10 to 14 mm, 57.9%; 16 to 22 mm, 69.9%; 22 to 26 mm, 73.9 %). Ectors et al. (197. Ectors FJ et al 1997) found that fertilization rates were greatest in follicles 16 to 23 mm in size on the day of oocyte retrieval (68%), compared with either those less than 16 mm (56%) or those more than 23 mm (56%). Oocyte maturation rates were more than 95.3% in those follicles more than 23 mm on the day of oocyte retrieval, as compared with 75.3% in those less than 16 mm (197. Ectors FJ et al 1997).
In 2016, Hu et al. (202) analyzed 492 IVF cycles treated with the short protocol and categorized patients by the proportion of follicles more than or equal to 10 mm on the day of trigger that were also more than or equal to 17 mm as low proportion (30% of follicles more than or equal to 10 mm were also more than or equal to 17 mm), middle proportion (30% to 60% of follicles more than or equal to 10 mm were also more than or equal to 17 mm), or high proportion (more than 60% of follicles more than or equal to 10 mm were also more than or equal to 17mm). The number of oocytes retrieved was greatest in patients with a low proportion of follicles more than or equal to 17 mm (oocyte number: low, 9.2; middle, 7.6; high, 7.2), suggesting that follicles more than 17 mm on day of trigger contribute less to the number of oocytes retrieved than do smaller follicles. And oocyte maturation rate was low (85%), middle (89%), and high (88%) (202. Hu et al 2016 )
Interval between trigger and oocyte retrieval
There is a continuum between the processes of oocyte maturation and ovulation if sufficient LH-like exposure is provided. Thus, it is critically important to schedule oocyte retrieval at a precise interval following administration of the agent of oocyte maturation, such
that it takes place following oocyte maturation, but prior to ovulation. If the interval is too long, ovulation may have occurred prior to retrieval and oocytes will no longer be present within the follicles, whereas if the interval is too short, insufficient time may have been provided for optimal oocyte maturation and oocyte retrieval is more difficult.
In the natural cycle, the median interval from the onset of rise in LH to ovulation is 32 hours (95% CI, 24 to 38) and following the peak in LH is 16.5 hours (95% CI, 10 to 23) (182. WHO 1980). Ovulation occurs in 90% of women between 16 and 48 hours after the first significant rise in LH and between 3 and 36 hours after the peak (182. WHO 1980).
Bosdou et al. randomised 156 normo-ovulatory women treated with the short protocol to either an interval of 36 hours or 38 hours following 250 mg of rhCG and oocyte retrieval and found no difference in oocyte retrieval rate, maturation rate (88% vs 80%) And no patient ovulated prematurely due to the extension of interval between hCG and oocyte retrieval (Bosdou et al)
In 2011, Wang et al. conduced a meta analysis of five trials investigating the interval between hCG and oocyte retrieval , including 895 patients. Oocyte maturation rate was significantly higher when oocyte retrieval was performed more than 36 hours after hCG administration, compared with less than 36 hours after administration .
Thus, there is likely to be significant inter individual variation in the interval between the LH surge and the time to ovulation in the natural cycle. Similarly, in stimulated cycles, there is evidence to suggest that extending the interval between hCG administration and oocyte retrieval beyond the standard 36 hours is unlikely to lead to premature ovulation.
Intra follicular changes following hCG, GnRHa triggers
Rosen MP et al. in 2009 (173) observed that intra follicular FSH levels were higher in follicles that yielded an oocyte. Lamb JD et al. (210) in 2010 observed that oocytes fertilized by ICSI were 28% to 35% more likely to be retrieved from follicles with higher intrafollicular concentrations of estradiol and testosterone, whereas oocytes fertilized by IVF were 9% to 14% more likely to arise from follicles with higher estradiol or progesterone concentrations.
Similarly, Itskovitz J et al. in 1991 (211) found that intrafollicular estradiol and progesterone levels were higher in follicles containing a mature oocyte. Interestingly, intrafollicular kisspeptin levels are higher than corresponding serum kisspeptin levels, and they correlate with follicular fluid estradiol levels and the number of mature oocytes retrieved (212. Taniguchi Y 2017).
In 2014, Haas et al. (213) assessed alterations in expression of genes related to steroidogenesis in granulosa cells of 24 women who received either GnRHa or hCG triggering. Expression of the enzymes CYP19A1 (0.50 vs 1) and CYP11A1 (0.6 vs 1), as well as 3beta-hydroxysteroid-dehydrogenase (0.39 vs 1), vascular endothelial growth factor (VEGF; 0.74 vs 1), and inhibin beta B (0.38 vs 1), was significantly lower in the GnRHa group (214. Inoue Y 2009 ). Expression of the FSH receptor was also significantly lower in the GnRHa group, but not expression of the LH receptor (213). Owens et al. (216) investigated expression of genes involved in ovarian reproductive function, steroidogenesis, and OHSS in granulosa lutein cells following the use of hCG, GnRHa, or kisspeptin to induce oocyte maturation in 48 women undergoing IVF treatment. Kisspeptin-54 increased expression of genes involved in ovarian steroidogenesis,
Similarly, hCG has been shown to directly increase VEGF expression and VEGF levels in human granulosa cells (217. Neulen J et al 1995, 219. Cerrillo M et al 2009, 220. Pellicer A et al 1999), whereas GnRHa may act directly on ovarian GnRH receptors to induce luteolysis (221. Casper RF et al 1979 , 222. Lemay A et al 1979).
Ovarian Hyperstimulation Syndrome
Ovarian hyperstimulation syndrome is an iatrogenic complication by an exaggerated response of ovarian stimulation in the aim to produce multiple follicles so as to increase the number of oocytes available.[1. Humaidan P et al 2010,17. Tan BK et al 2013] Severe OHSS is a potentially life-threatening, grave complication and occurs in about 2%-6% of IVF cycles resulting in hospitalization in about 1.9% of cases.[18. Mocanu E et al 2007] It is associated with massive ovarian enlargement, shift of protein-rich fluid from the intravascular space to the third space (abdominal, thoracic cavity), liver dysfunction, electrolyte imbalance resulting in significant morbidity and rarely, mortality due to thromboembolic disease, adult respiratory distress syndrome, and hepatorenal failure.[1.Humaidan P et al 2010 ,4. Papanikolaou EG 2006]
The main culprit triggering the syndrome is the presence of hCG [19] either exogenous or endogenous. Exogenous hCG administration results in early OHSS, an acute event usually occurring within 9 days after oocyte retrieval.[19. Lyons CA et al 1994,20. Mathur RS et al 2000] Late OHSS occurs after the initial 10-day period and is due to the endogenous hCG produced by an implanting embryo [4. Papanikolaou G et al 2006 ,20. Mathur RS et al 2000] or the administration of hCG for luteal phase support. hCG increases the ovarian vascular permeability and is responsible for activating the VEGF pathway resulting in OHSS. Prostaglandins, inhibin, renin-angiotensin-aldosterone system, insulin-like growth factor 1, interleukin-6, and other inflammatory mediators have all been implicated in the etiology of OHSS. [21. Pellicer A et al 1997] However, VEGF has been identified as the major mediator.[22. Neulen J et al 1995] In addition, hCG has been shown to increase VEGF expression in the human granulosa cells, which in turn raises serum VEGF concentration thereby increasing the severity of OHSS.[21. Pellicer A et al 1997,22. Neulen J et al 1995]
Exogenous hCG has been used to induce final follicular maturation in all IVF cycles as both hCG and LH bind to and activate the same receptor, LH receptor (LHR) which promotes follicular maturation, ovulation and luteinization.[23.Ascoli M et al 2002] However, the hCG molecule has a high biological activity, which is about 6–7 times higher than the endogenous LH with a half-life exceeding 24 h, while, the half-life of LH is 60 min. hCG has a greater affinity to LH receptor as compared to LH, and thus exerts a more prolonged luteotrophic action for 8–9 days, multiple corpus luteum development and raised serum levels of E2 and P4 throughout the luteal phase,[3] all of which increase the risk of developing OHSS.[6] This risk is similar for both urinary derived and rhCG.[24. Al-Inany HG et al 2005] Of the various strategies enumerated for the prevention of OHSS, the most effective strategy to date is the use of GnRHa as trigger in antagonist cycles, which virtually eliminates its occurrence as proven in various studies.[1,11,12. Humaidan P et al 2011,13. Engmann L et al 2008,14. Kol S et al 2004,15. Humaidan P et al 2014,16. Griesinger G 2011]
Itskovitz et al. in 1988,[44] first introduced the concept that GnRHa trigger eliminates OHSS in high-risk patients, 10 years even before the GnRH antagonist era. With GnRH antagonists becoming more clinically in usage, GnRHa have gained much interest and has become possible to trigger final oocyte maturation and ovulation as an alternative to hCG.[12. Humaidan P et al 2011,45]
The GnRHa displaces the antagonist from its receptor, activating the receptor, which causes a flare-up effect, inducing gonadotropin release. The GnRHa-induced surge, although effectively stimulates ovulation and oocyte maturation, there exist differences in regards to its duration and profile when compared to that of the natural cycle LH surge.[12. Humaidan P et al 2011,45] The GnRHa-induced LH surge consists of two phases: A short ascending limb ( about 4 h) and a long descending limb ( about 20 h), with a total duration of 24–36 h.[12. Humaidan P et al 2011] While, the LH surge of the natural cycle is characterized by three phases: A rapidly ascending phase of 14 h, a plateau phase lasting for 14 h, and a descending phase of 20 h, in total of 48 h.[12. Humaidan P et al 2011] Thus, the total amount of gonadotropins released with GnRHa trigger is significantly reduced when compared to natural cycle. The shorter duration of the endogenous LH surge induced by GnRHa trigger and the rapid demise of the corpora lutea by withdrawal of LH support reduces the risk of OHSS.[12,15,16,45] VEGF proves to be significantly lower in patients receiving GnRHa instead of hCG, both at the messenger RNA and glycoprotein levels of VEGF are low .[12]
In segmentation strategy,[46. Devroey P et al 2011] of freezing all embryos as accompanying supraphysiologic steroid levels impairs the endometrial receptivity, as well as being embryo-toxic.[50]
In this study, although the number of DFs, IMFs, and peak E2 on the day of trigger (12.74 ± 4.3 vs. 10.91 ± 2.9), (11.7 ± 3.9 vs. 9.9 ± 3.3), and (4878.1 ± 2531 vs. 3870.4 ± 1556), respectively was all higher in the study group as compared with the hCG group [Table 2], yet none developed moderate or severe OHSS (0% in GnRHa group vs. 37.6% in hCG group) [Table 3]
In concordance, two randomized studies in patients at risk of OHSS by Babayof et al.[51] and Engmann et al.[13] reported an incidence of 31% OHSS in the hCG group versus 0% in the GnRHa trigger group.
Similar results were obtained by Griesinger et al.[52] and Manzanares et al.[53] in PCOS and high responders with an incidence of 0% OHSS in the GnRHa group. Cochrane review showed that the GnRHa was associated with lower risk of OHSS (mild, moderate, or severe) than hCG (eight RCTs, odds ratio [OR]: 0.15, 95% CI: 0.05–0.47) in fresh autologous cycles.[47]
In a recent systematic review and meta-analysis by Youssef et al. 2015,[54] GnRHa seem to be safer than traditional hCG due to the associated low risk of OHSS in fresh autologous cycles (ten RCTs, OR: 0.06, 95% CI: 0.02–0.19).[54]
However, majority of studies evaluated the role of GnRHa in normoresponders with normal to low risk of developing OHSS. The role of agonist trigger in terms of OHSS incidence was further elucidated used the donor-recipient model in randomized clinical trials and none of the patients developed OHSS whereas the OHSS incidence after hCG triggering was between 4% and 17%.[55. Melo M et al 2009 ,56.Sismanoglu A et al 2009]
Cochrane review also confirmed a lower incidence of OHSS in the donors triggered with GnRHa group than in the hCG group (three RCTs, OR: 0.05, 95% CI: 0.01–0.28).[47.Youssef MA et al 2014]
In addition, the use of GnRHa trigger has resulted in higher patient convenience during the luteal phase, with less abdominal distension and discomfort as reported by Hernández ER et al in 2009 [57] and Cerrillo M et al in 2009 [58] The patients in this study who were followed up on day 4 and 7 following oocyte retrieval were totally asymptomatic and almost none had nausea, vomiting, abdominal distension/pain in the GnRHa group. Only one patient in the GnRHa group manifested with nausea and mild abdominal discomfort on the 3rd day postoocyte retrieval and settled with symptomatic treatment. On the contrary, in the hCG group, 35 patients had moderate OHSS, 13 of whom required hospitalization for 3–5 days and 3 patients (2.9%) had severe OHSS requiring intensive care in the hospital for 5–7 days.
With GnRHa trigger and fresh embryo transfer, the addition of hCG with standard luteal phase support to improve implantation and clinical pregnancy results in an increased risk of OHSS.[59. Seyhan A et al 2013] A high incidence of early-onset OHSS (22%) after GnRHa trigger was reported in a retrospective study in a population of high-responder patients who received a low-dose hCG rescue protocol. Here, the investigators proceeded with modified luteal phase protocol instead of a freeze-all policy, despite retrieval of as many as 50–65 oocytes in some patients.[59. Seyhan A et al 2013]
In a similar study by Datta AK et al. in 2014 [60] an incidence of mild-to-moderate OHSS of 16.2% with GnRHa trigger and 31.0% with hCG trigger in high responders with the addition of a single hCG dose of 1500 IU in the luteal phase was reported. However, this again was a retrospective study with a small sample size (GnRHa [n = 62]; hCG [n = 29]). On the contrary, an RCT in a high-risk population consisting of patients with a follicle count between 15 and 25 did not show any OHSS (0% in GnRHa vs. 2% in hCG), despite the use of the aforementioned low-dose hCG 1500 IU rescue in the luteal phase.[2. Humaidan P et al 2013, 45] Thus, the need to set an upper cutoff limit of follicles for the use of hCG in luteal support in fresh transfer and freeze all policy after GnRHa trigger have been commented by Humaidan et al.[2. Humaidan P et al 2013] In the literature, two cases of severe OHSS after GnRHa trigger in GnRH antagonist protocol without the administration of any hCG for luteal phase support possibly due to GnRH receptor, FSH receptor, or LH receptor gene mutations has been reported.[61]
A possible advantage of the GnRHa-induced surge over the hCG-induced surge is the simultaneous induction of a surge of FSH resembling that of a natural cycle. Although the exact role of this mid-cycle FSH surge has not been clear, it has been shown that it promotes nuclear maturation with the resumption of meiosis.[12. Humaidan P et al 2011, ] This might explain the retrieval of more metaphase oocytes after GnRHa trigger compared with hCG trigger.[11,12. Humaidan P et al
2011,44. Itskovitz J et al 1988 ,45] The retrieval of more mature oocytes in the GnRHa triggered group in this study when compared to the hCG group [Table 4] supports the previous clinical findings of a possible beneficial effect of the mid-cycle FSH surgeon oocyte maturity
Current Modes to Induce Final Oocyte MaturationhCG
Both hCG and LH are complex heterodimeric glycoproteins with high cystine content. hCG has structural similarity to LH, sharing the same a subunit and 85% of the amino acid structure of the b subunit (81. Hershko klement et al 2017). This property affords hCG the ability to stimulate the LH receptor (4) and to induce luteinization of granulosa cells and the resumption of meiosis (82. Orvieto R et al 2017).
Although hCG activates the LH receptor, it does not do so in an identical manner to LH. Roess et al. (83. Roess DA et al 1997) demonstrated differences in the binding of LH and hCG to the LH receptor by rotational diffusion. Receptors bound by hCG were immobile, whereas those bound by LH were rotationally mobile, potentially accounting for differences in receptor activation (83. Roess DA et al 1997). hCG is 30 % carbohydrate by weight and has greater glycosylation than does LH, which may also account for differences in receptor binding (83). Furthermore, intracellular signaling following activation of the LH receptor differs depending on the ligand bound (83). hCG possesses higher affinity for the LH receptor than LH, and it is fivefold more potent in stimulating human granulosa cell cAMP activity than equimolar concentrations of LH (66). However, extracellular signal-related kinase 1/2 and AKT (protein kinase B) activation is greater following LH than hCG (84. Casorini L et al 2012).
In summary, hCG has a greater effect on cAMP and steroidogenic action than does LH, whereas LH has a greater effect on extracellular signal-related kinase 1/2 and AKT signaling, which are anti apoptotic proliferative signals. This difference in action is hypothesized to relate to their physiological roles in the normal menstrual cycle and in early pregnancy, whereby LH plays a key role in inducing oocyte maturation and ovulation, whereas hCG supports the developing embryo and decidua through stimulating steroidogenesis. The translation of these in vitro findings is further complicated by the presence of a complex hormonal milieu in vivo that can alter these in vitro behaviors (85. Casarini L et al 2017). In conclusion, although both LH and hCG activate the LH receptor, they are not equivalent with regard to both their receptor binding kinetics and the intracellular signaling that they induce.
Urinary or Recombinant Human Chorionic Gonadotrophin ?
For decades, the only formulation of hCG was derived from the urine of pregnant women (86). However, urinary hCG (uhCG) may contain significant batch-to-batch inconsistencies, and it has the potential for immunological reactions, prions and impurities (86. European r HCG study group 2000). The advent of recombinant DNA technology has made it possible for recombinant hCG
(rhCG) to be synthesized in Chinese hamster ovary cells without the need for any human resource, thereby limiting the above issues (87. Loumaye E et al 1996,88. Driscoll GL et al 2000). uhCG is usually administered intramuscularly, whereas rhCG can be administered subcutaneously.
Equivalence between uhCG and rhCG was demonstrated in a phase III double-blinded, randomized controlled study by Driscoll et al. (88.Driscoll GL et al 2000). The authors compared subcutaneous administration of 250 mcg of rhCG with intramuscular administration of 5000 IU of uhCG in 84 p women, and they found no significant differences in the number of oocytes retrieved (rhCG 10.8 vs uhCG 10.3), the number of oocytes retrieved per follicle >10 mm on day of trigger (rhCG 90% vs uhCG 80%), the number of mature oocytes (rhCG nine vs uhCG eight), or the number of cleaved embryos (88. Driscoll GL et al 2000).
A larger randomized controlled study of 297 patients confirmed equivalence, with a similar number of oocytes retrieved following 10,000 IU of uhCG, 250 mcg of rhCG, or 500 mcg of rhCG (89).
In 2017, Bagchus W et al. (90) compared 250 mcg (~6500 IU) of rhCG subcutaneously with 10,000 IU of uhCG intramuscularly in Japanese and white women (90). Maximal hCG concentrations occurred between 16 and 32 hours before declining during 11 days following administration (90). Interestingly, the mean exposure and mean maximum concentration (C max) following rhCG was about 20 % lower in Japanese women than in white women (90). In Japanese women, C max was higher following uhCG than rhCG (141 vs 126 IU/L) and occurred sooner [time to maximum concentration (t max) 18 hours vs 22 hours] (90). The half-life was similar at approximately 35 hours following both preparations and in both ethnicities (90). However, importantly white women chosen for this study were weight-matched to Japanese women with a mean weight of 52 kg, and thus they were not chosen to exemplify a representative white population with higher body weights. To investigate the possibility that urinary hCG may contain additional factors, such as epidermal growth factor (EGF), that could negatively influence trophoblast function, in 2010 Papanikolaou EG et al. (91) randomized 119 women to receive either 250 mcg of rhCG or 10,000 IU of uhCG to assess pregnancy rates. Live birth rate per protocol was higher following rhCG compared with uhCG (44.1% vs 25.7%) owing to an increased rate of early miscarriage in the uhCG group (28.0% uhCG vs 3.5 % rhCG, P = 0.01) (91). The authors hypothesized that rhCG may have beneficial effects on placentation compared with uhCG, or that an embryonic factor could account for the difference (91.Papanikolaou EG et al ). However, the superiority of rhCG over uhCG has yet to be demonstrated, and two recent Cochrane reviews have found no difference in the rates of oocyte maturation, pregnancy outcomes, or OHSS (92. Youssef M et al 2016 ).
Optimum Dose of Recombinant hCG
When the lower doses of HCG can to induce oocyte maturation and granulosa cell luteinization, they may be insufficient to ensure optimal cytoplasmic oocyte maturation for fertilization and corpora lutea function (93. Zelinski wooten MB et al 1997). Hence, a higher dose of hCG influences both the amplitude of hCG level attained, as well as the duration at which hCG levels are maintained over a threshold value. The terminal half-life of rhCG is estimated to be 29 +/- 4.6 hours (21. Mann K et al 1980, 22. Norman RJ et al 2000), compared with a half-life of around 30 minutes for endogenous LH (94. Santen RJ et al 1973).
Although 500 mcg of rhCG resulted in two more zygotes/cleaved embryos than did the lower dose of 250 mg rhCG, this came at the expense of an increased rate of OHSS (9% vs 3%) (89). Thus, 250 mcg of rhCG was recommended for clinical use, being more convenient to administer than uhCG and causing lower rates of OHSS than the higher dose of rhCG.
Matorras et al. in 2012 (105) investigated serum hCG levels at 36 hours following 250 mcg of rhCG subcutaneously in 473 women, again demonstrating that serum hCG negatively correlates with BMI (serum hCG = 196 – 3.9 * BMI; r2 = 0.14) (105). Patients with no oocytes retrieved had a lower serum hCG (77mIU/mL) compared with those with at least one oocyte retrieved (more than 116 mIU/mL) (105). The mean number of oocytes retrieved was similar by categories of serum hCG level (84 oocytes recovered even in those less than 50 mIU/mL at 36 hours) and oocyte recovery rates were similar across hCG levels (105). This suggests that although most will have effective oocyte maturation with a standard dose, some individuals with higher BMI could benefit from higher hCG doses to ensure efficacious triggering.
GnRH agonist Trigger
Although the ability of GnRH agonists to trigger oocyte maturation has been recognized since the 1970s (Nakano R et al 1973 ), their potential to induce oocyte maturation was fully realized with the advent of the competitive reversible GnRH antagonists in the 1990s (107). GnRH adisplace the GnRH antagonist from the GnRH receptor, leading to receptor activation and gonadotropin release from the pituitary gland (106. Nakano R et al 1973 ).
Schally et al. (108,109.Schally AV in 1999) first isolated GnRH (at the time more commonly referred to as “luteinizing hormone-releasing hormone”) and synthesized analogs by substituting one or more of the 10 amino acids. Replacement in position 6 or 10 resulted in the formation of analogs that were both more potent than endogenous GnRH and had greater duration of action at the GnRH receptor, with examples including triptorelin, leuprolide, and buserelin (109, 110. Coy DH 1976, 111. Vickery BH 1986). The half-life of endogenous GnRH is around 2 to 4minutes; however, the half-life of GnRHa is extended (4,112. Sennello LT 1986 ) according to amino acid replacement, for example, triptorelin half-life (t 1/2 ) about 4 hours, nafarelin t 1/2 3 to 4 hours, leuprolide t 1/2 1.5 hours, and buserelin t 1/2 1.3 hours (25). The endogenous LH surge lasts around 48 hours and consists of three distinct phases (113), whereas LH secretion following GnRHa is characterized by two phases, that is, a rapid ascending phase lasting 4 hours, and a longer descending phase (114. Itskovitz J et al 1991) (see Fig. 3 for a diagram of hormonal profiles following agents used to induce oocyte maturation). Of relevance, GnRH activates pituitary GnRH receptors to release both endogenous LH and FSH, whereas hCG possesses only LH-like activity (115. Ben Arie A 1996). Whereas the mid-cycle FSH surge is not critical for oocyte maturation to occur, FSH is known to increase LH receptor expression in granulosa cells and additionally may directly play a role in the expansion of cumulus–oocyte complexes and oocyte maturation (116, 117. Atef A et al 2005, 118. Eppig JJ 1979).
GnRHa preparations and dosing regimes
A number of GnRHas have been used to trigger oocyte maturation, with the literature encompassing different agonists and dosages. Several studies have used buserelin at 0.5 mg (23, 24) whereas triptorelin is frequently prescribed at 0.2 mg (27,28). A recent RCT demonstrated no difference in LH profiles, the number of mature oocytes, fertilization rates, or embryogenesis in oocyte donation cycles following doses of triptorelin between 0.2 mg, 0.3 mg, and 0.4 mg, suggesting that 0.2 mg is at the upper end of the dose-response range (26).Leuprolide acetate has been used at doses ranging from 0.5 mg (29) to 4 mg (31).
Which GnRH agonist is the best for Oocyte maturation?
In 2017, S¸¨uk¨ur YE et al. retrospectively compared patients who received triptorelin at 0.2 mg (n = 63), leuprolide at 1 mg (n = 74), or 10,000 IU of hCG when serum estradiol was less than 3000 pg/mL (n = 113) (120). The efficacy of oocyte maturation appeared similar between the interventions; the number of mature oocytes divided by the number of follicles more than 14 mm on the day of oocyte retrieval (calculated on aggregated data) was 120 % for triptorelin, 142 % for leuprolide, and 128% for hCG (120).
Parneix I et al. in 2001 (119) compared 231 women undergoing ovulation induction with 1 of 13 different regimens for inducing ovulation, including triptorelin, buserelin (both intranasally and subcutaneously), leuprolide, naforelin, or hCG. The authors reported that all regimens resulted in ovulation with no evidence of superiority of one analog over another, and with similar pregnancy rates between groups and the control (hCG) group (119).
Thus, at present, although dosing and type of GnRHa vary in the literature, there is insufficient evidence to support preferential use of any GnRHa over another (4).
Efficacy of GnRHa as compared with hCG
In 2010, Oktay et al. (n = 47) compared leuprolide acetate at 1 mg (n = 27) and hCG at 5000 to 10,000 IU to induce oocyte maturation in women undergoing fertility preservation treatment (103). Although the total number of oocytes was similar between the two groups (GnRHa 16.4 , hCG 12.8), a greater number of mature oocytes (GnRHa 11.9, hCG 7.4 ; P less than 0.001), higher fertilization rate (GnRHa 84.1%, hCG 74.0%; P = 0.027), and more zygotes were observed following GnRHa (103). A prospective randomized controlled study of patients by Humaidan et al. (24) reported that although significantly more oocytes were retrieved following 10,000 IU of uhCG (9.7) than following
buserelin at 0.5 mg (8.4), the oocyte maturation rate was higher following buserelin (84% vs 68%), leading to slightly more mature oocytes. Nonetheless, luteal levels of progesterone and estradiol were lower in the GnRHa group, corresponding to reduced implantation (GnRHa 3 of 89, hCG 33 of 97 ) and clinical pregnancy rates (GnRHa 6%, hCG 36%), with increased early pregnancy loss (GnRHa 79%, hCG 4%) (24). In an early meta-analysis, including three studies (104), GnRHa was determined to have similar efficacy to hCG with regard to the number of mature oocytes retrieved, oocyte maturation rate, fertilization rate, and embryo quality.
Thus, GnRHa induces a gonadotropin surge sufficient for oocyte maturation, but it induces a shorter duration of LH exposure than hCG. Whereas this affords an improved safety profile, the same property results in a smaller chance of functional corpora luteal and an increased emphasis on adequate luteal phase support to maintain pregnancy rates.
The advantages of using GnRH-a in the final oocyte maturation
In some studies, the use of GnRH-a in the final oocyte maturation has similar or better results compared to hCG trigger (7-11). Unlike hCG trigger, GnRH-a trigger stimulates FSH surge in addition to LH surge. FSH surge, in the mid-cycle, has a specific effect on oocyte maturation and leads to a further expansion of cumulus cells surrounding the oocyte and release of proteolytic enzymes involved in the process of ovulation(12-15). Lamb et al by adding a dose of FSH to the hCG trigger, showed better recovery of oocyte and higher fertilization rates in IVF compared with hCG trigger alone (16). Another advantage of this method is more maturity of the nucleus and the resumption of meiosis and eventually increasing the number of Metaphase II oocytes (9, 10, 17-19). In addition, increased levels of LH following injection of hCG is slower than that following GnRH-a trigger (20). Overall, GnRH-a trigger with effects of FSH along with the LH in the final follicular maturation, may result a more physiological maturity. Likely, more maturity of oocyte might be related to increase faster in LH surge compared with an increase of LH after 10000 IU IM injection of hCG and also a concomitant increase of FSH (21).
GnRH-a decreases significantly the risk of OHSS and gradually is used in most clinics to induce final oocyte maturation in patients with the risk of OHSS (22).
Recombinant LH trigger :
In the mid-1990s, rLH became available as a further therapeutic option for use in IVF treatment. Following intravenous administration, rLH has a similar pharmacokinetic profile to endogenous LH with a distribution half-life of about 1 hour and a terminal half-life of 10 to 12 hours (32, 33, 123). Peak serum LH levels were attained 4 to 5 hours following subcutaneous administration of 10 000 IU of rLH with a terminal half-life of about 22 hours (33).
In 2001 a multi-center trial across 22 centers in nine countries was conducted to investigate the efficacy of rLH to induce oocyte maturation in comparison with uhCG (15). Two hundred fifty women treated with the long protocol had final oocyte maturation induced by either subcutaneous rLH at doses of 5000 IU (n = 39), 15,000 IU (n = 39), 30,000 IU (n = 56) (15,000 IU plus 10,000 IU administered 3 days after the first injection; n = 25), or intramuscular uhCG at 5000 IU (n = 121) (15). Mean serum LH levels at 24 hours following rLH increased dose-dependently from 23.3 IU/L following 5000 IU to 93 IU/L following 30,000 IU (15).The number of oocytes and zygotes following rLH increased dose dependently by approximately one per dosing category (15). The estimated mature oocyte yield using aggregated data (number of mature oocytes/number of follicles 10 mm) was 70 % following 5000 IU of rLH, 77 % following 15,000 IU of rLH, and 87 % following 30,000 IU of rLH as compared with 68 % to 75 % following uhCG (15). Two patients had no oocytes retrieved despite comparable serum LH levels (one in the 30,000 IU rLH group and another in the 15,000 IU rLH group). The oocyte maturation rate did not show a clear dose-response
and was indeed worse at the highest dose of rLH (15).
A lack of corresponding FSH response may account for some of the reduced efficacy of oocyte maturation in comparison with GnRHa at comparable LH levels. The exact profile of the LH levels achieved and in particular the rise in LH during the first 24 hours was not clear from the data collected, and thus the time to oocyte retrieval may not have been optimal. However, no clear advantage was observed from the use of rLH over hCG and very large doses were required to achieve efficacy. Thus, rLH is not currently in clinical use as an inductor of oocyte maturation. Oral LH agonists are also in development and have been investigated in ovulation induction cycles, but have yet to be evaluated during IVF cycles.
Kisspeptin
Kisspeptin, named after Hershey’ kisses chocolates, is a hypothalamic neuropeptide that results in gonadotropin release in both men and women and is requisite for ovulation in women (53. Dhillo WS et al 2007 ,54. Clarkson J et al 2008 ). The sensitivity to kisspeptin increases during the preovulatory phase when estradiol levels are highest (53. Dhillo WS et al 2007). Kisspeptins are a group of hypothalamic arginine-phenylalanine amide peptides encoded for by the KISS1 gene on chromosome 1q32 (126. Tena sampere M 2006). kisspeptin acts on the hypothalamus to stimulate the release of endogenous GnRH and subsequent gonadotropin release. Their activity at the G protein–coupled kisspeptin receptor is conferred by a common C-terminal decapeptide sequence, equivalent to kisspeptin-10 (127. Ohtaki T et al 2001 ,128. Kotani M et al 2001). Kisspeptin acts at the kisspeptin receptor on GnRH neurons in the hypothalamus to elicit endogenous GnRH release. The first trial evaluating the use of kisspeptin-54 to induce oocyte maturation was undertaken in 2014 using an adaptive design (18). Single doses of kisspeptin-54 at 1.6 nmol/kg (n = 2), 3.2 nmol/kg (n = 3), 6.4 nmol/kg (n = 24), and 12.8 nmol/kg (n = 24) were administered 36 hours prior to oocyte retrieval following a standard short protocol (18). Peak plasma levels of kisspeptin were observed at 1 hour following subcutaneous administration resulting in mean serum LH levels of 37.1 IU/L following 6.4 nmol/kg of kisspeptin, and 42.1 IU/L following 12.8 nmol/kg of kisspeptin at 4 to 6 hours following administration with serum LH levels tending toward baseline levels at 12 to 14 hours following administration (18).
To date, the trials using kisspeptin suggest that it could be a promising future option particularly in the woman at high risk of OHSS; however, further trials directly comparing kisspeptin to current modes of inducing oocyte maturation are required.
Double versus Dual triggers
When GnRH agonist and HCG are given simultaneously it is termed as “dual trigger”
when GnRH agonist is given first, and hCG is given later to rescue the luteal phase is termed as “double trigger”.
If there is no LH surge and/or progesterone rise after GnRHa trigger, repeat trigger with hCG and oocyte retrieval 35 hours later have been shown to result in successful retrieval of oocytes (54). If there is a suboptimal LH rise with values less than 15 IU/L, repeat trigger with hCG can be given as soon as possible to proceed with retrieval as planned or the cycle may be cancelled. Alternatively, the patient can proceed with unilateral follicle aspiration and, if there are no oocytes, re-trigger with hCG and repeat oocyte retrieval of the contralateral ovary 34 hours later (64).
Addition of a standard or low-dose hCG to GnRHa trigger in a “dual trigger” protocol demonstrated an improvement in the number and proportion of mature oocytes (73), and has been adopted for wide use in some clinics to reduce the chances of EFS with GnRHa trigger (33).
In 2014, Haas et al. (158) conducted a pilot study assessing whether patients with a low oocyte yield (defined as the ratio of the number of oocytes retrieved divided by the number of follicles more than 14 mm on day of trigger of less than 50%) in response to hCG could be improved by the addition of a GnRHa and increasing the interval to oocyte retrieval. Eight patients with a low oocyte yield following hCG at 250 mcg administered 36 hours prior to oocyte retrieval subsequently received 0.2 mg of triptorelin 40 hours prior and hCG at 250 mcg 34 hours prior to oocyte retrieval (158). The number of oocytes retrieved improved from 2.3 to 7, the number of oocytes from follicles more than 10 mm improved from 19% to 80%, and the number of oocytes from follicles more than 14 mm improved from 19% to 118%, with three of eight patients having ongoing pregnancies (15&).The same group showed a similar improvement in 12 patients with low oocyte maturation rates (less than 66%) (174. Zilberbeg E et al 1015).
Again, improvements were seen in the number of oocytes with double trigger (10.4 vs 8.8), the number of mature oocytes (6.5 vs 3.7), the oocyte maturation rate (70% vs 47%), and the ongoing pregnancy rate (50% vs 0%) (174. Zilberbeg E et al 2015 ). Since two interventions were investigated simultaneously, it is difficult to identify which intervention resulted in the improvements observed. Although there is biological plausibility to the addition of GnRHa providing additional LH and FSH exposure, and several other non randomized studies have reported that patients with poor oocyte maturation in a first cycle triggered with hCG can have improved out comes in a subsequent cycle if supplemented with GnRHa (76, 175. Griffin D et al 2014 ,176. Fabris AM et al 2017 ).
Results and Analysis
|
Group |
Frequency |
Percentage |
|
Triptorelin Acetate Group |
53 |
49.5 |
|
Recombinant HCG Group |
54 |
50.5 |
|
Total |
107 |
100.0 |
|
Age-wise distribution of the Participants |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
22 |
34 |
29.17 |
3.185 |
0.089 |
|
Recombinant HCG Group |
23 |
38 |
30.24 |
3.262 |
The above table shows that the mean age of the Triptorelin Acetate Group and Recombinant HCG groups were 29.17 vs 30.24 respectively and was found to be statistically Insignificant
Age-category of the participants
|
Age Category |
Triptorelin Acetate Group |
Recombinant HCG Group |
||
|
Frequency |
Percentage |
Frequency |
Percentage |
|
|
21-25 years |
9 |
17.0 |
7 |
13.0 |
|
26-30 years |
24 |
45.3 |
19 |
35.2 |
|
31-40 years |
20 |
37.7 |
27 |
50.0 |
|
36-40 years |
0 |
- |
1 |
1.9 |
|
Total |
53 |
100.0 |
54 |
100.0 |
The above table shows the age distribution of two groups Triptorelin acetate Trigger and Recombinant HCG trigger groups.In Recombinant HCG Trigger group 7 patients were 21-25 years, 17 patients were 26-30 years and 27 patients were 31 to 35 years . And in Triptorelin Trigger group 9 patients were 21-25 years 24 patients were 26-30 years and 20 patients were 31-35 years. The mean value was 30.24+|- 3.23 in the Recombinant HCG Trigger group and 29.17 +|- 3.15 in the Triptorelin Trigger group. The P value was 0.0858.
|
AMH of the Participants |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
2 |
4 |
3.13 |
.833 |
0.902 |
|
Recombinant HCG Group |
2 |
4 |
3.11 |
0.925 |
The above table shows the AMH level of the participants in both trigger groups. The AMH value in the Recombinant HCG Trigger group, 25 patients had AMH 1.6-2 ng/ml, 16 patients had 2.1-2.5 ng/ml, 5 patients had 2.6-3.0 ng/ml, 4 patients had 3.1-3.5 ng/ml and 2 patients had 3.5-4 ng/ ml. In the Triptorelin group, 9 patients had 1.6 to 2 ng/ ml, 6 patients had 2.1-2.5 ng/ml, 8 patients had 2.6 to 3.0 ng/ml 18 patients had 3.1 to 3.5 ng/ml and 13 patients had 3.6 to 4 ng/ml The mean value was 2.21+|- 0.60 in the Recombinant HCG Trigger group and 3.02+|- 0.7 in Triptorelin Trigger group. The P value was < 0.0001.
|
D2AFC of the Participants |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
8 |
24 |
19.06 |
4.483 |
0.135 |
|
Recombinant HCG Group |
8 |
25 |
17.76 |
4.429 |
The above table shows the Antral Follicular Count on Day 2 of cycle for the participants in both groups
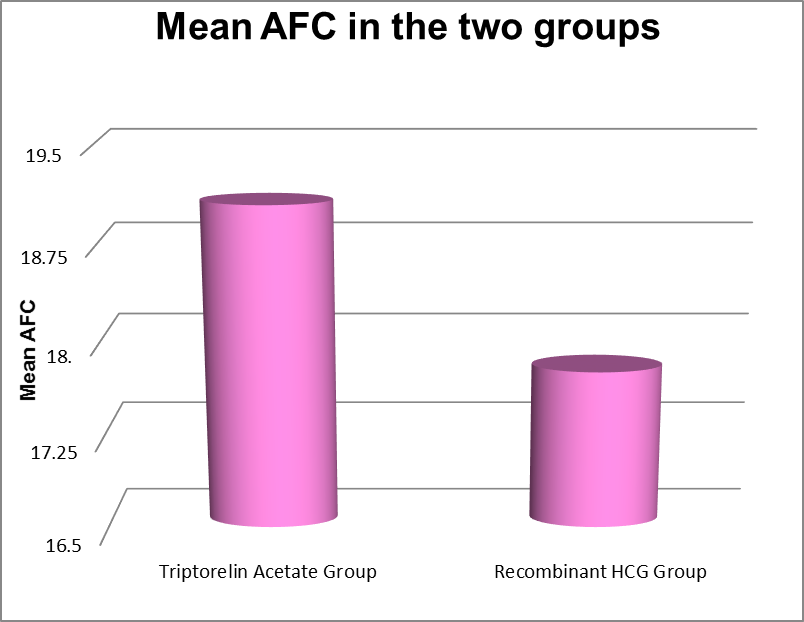
|
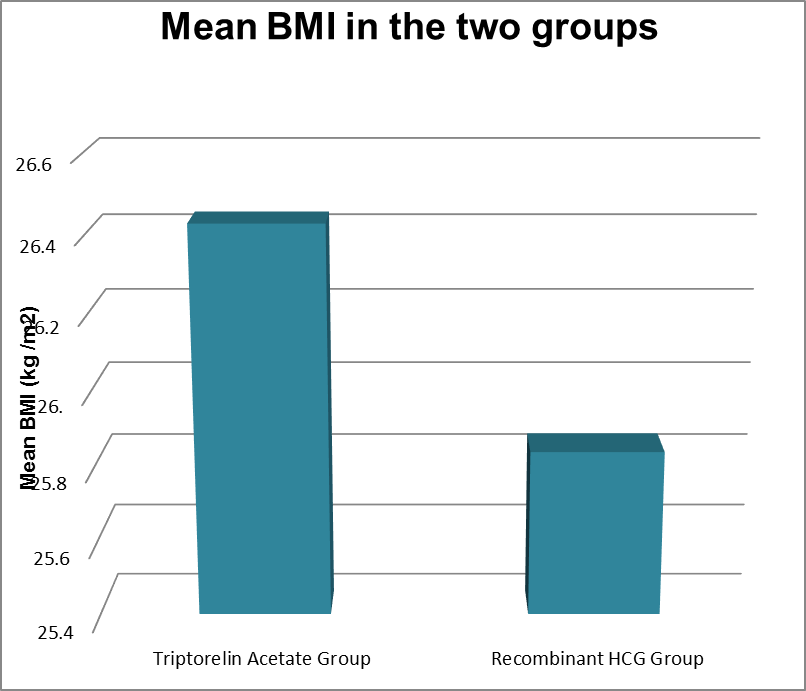
BMI of the Participants |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
21.8 |
29.3 |
26.43 |
2.14 |
0.172 |
|
Recombinant HCG Group |
19.0 |
29.0 |
25.84 |
2.29 |
|
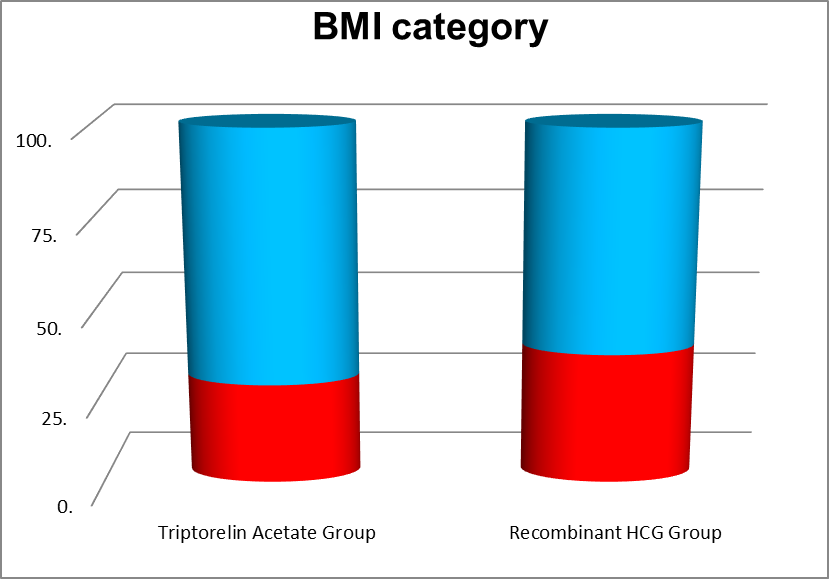
BMI Category (kg / m2) |
Triptorelin Acetate Group |
Recombinant HCG Group |
||
|
Frequency |
Percentage |
Frequency |
Percentage |
|
|
Normal BMI (18.5 – 25) |
15 |
28.3 |
20 |
37.0 |
|
Overweight (25.1 – 30) |
38 |
71.7 |
34 |
63.0 |
|
Total |
53 |
100.0 |
54 |
100.0 |
|
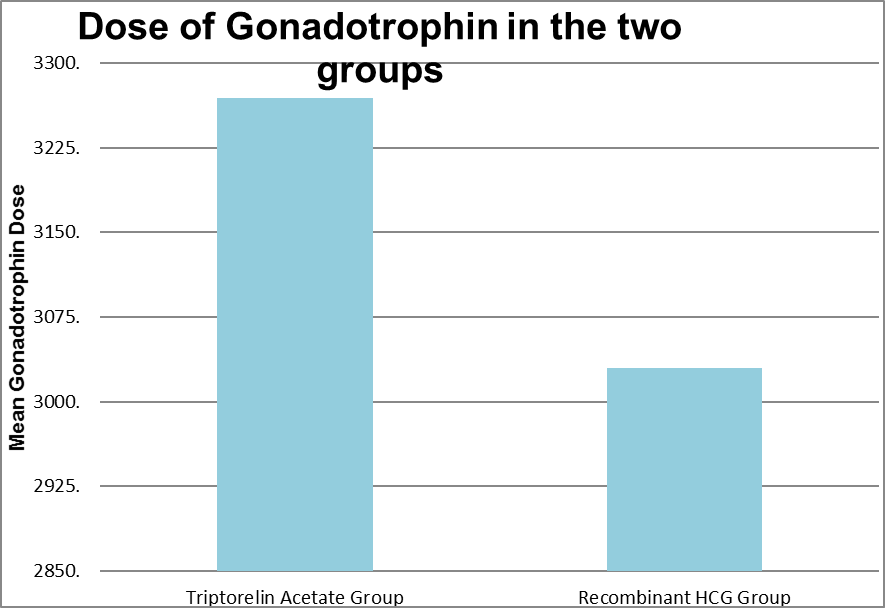
Total dose of Gonadotrophin IU |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
2125 |
6375 |
3269.19 |
737.159 |
0.171 |
|
Recombinant HCG Group |
-3125 |
4500 |
3029.94 |
1029.752 |
|
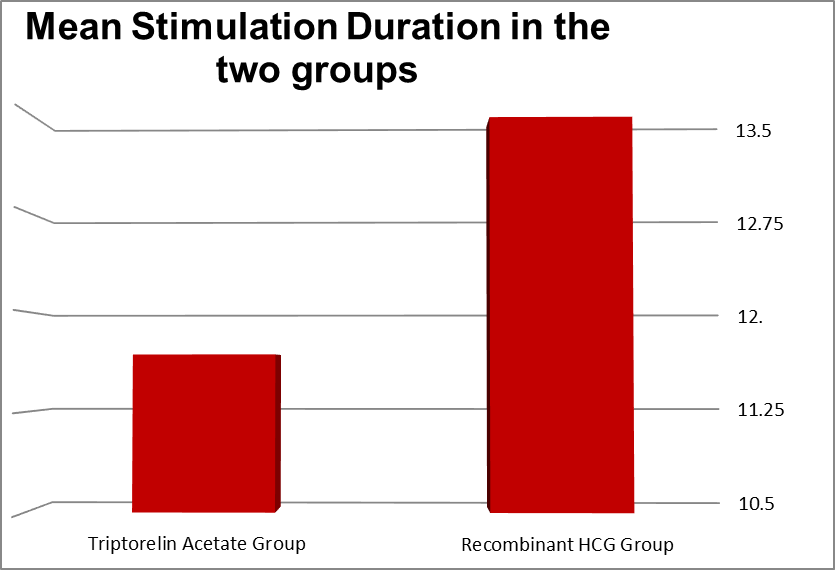
Duration of stimulation ( Days) |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
10 |
13 |
11.68 |
0.95 |
0.341 |
|
Recombinant HCG Group |
9 |
111 |
13.46 |
13.54 |
|
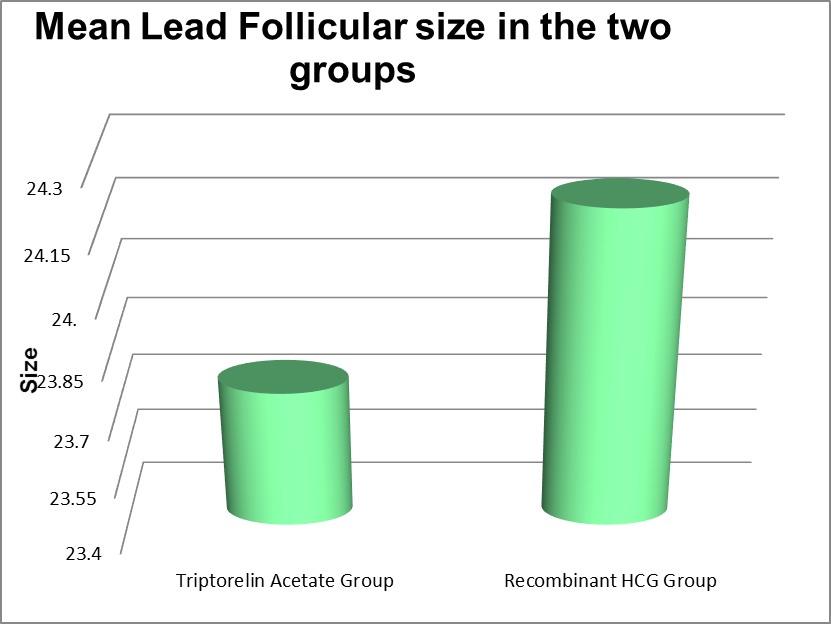
Lead Follicular Size |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
13 |
26 |
23.75 |
1.65 |
0.062 |
|
Recombinant HCG Group |
24 |
26 |
24.20 |
0.56 |
|
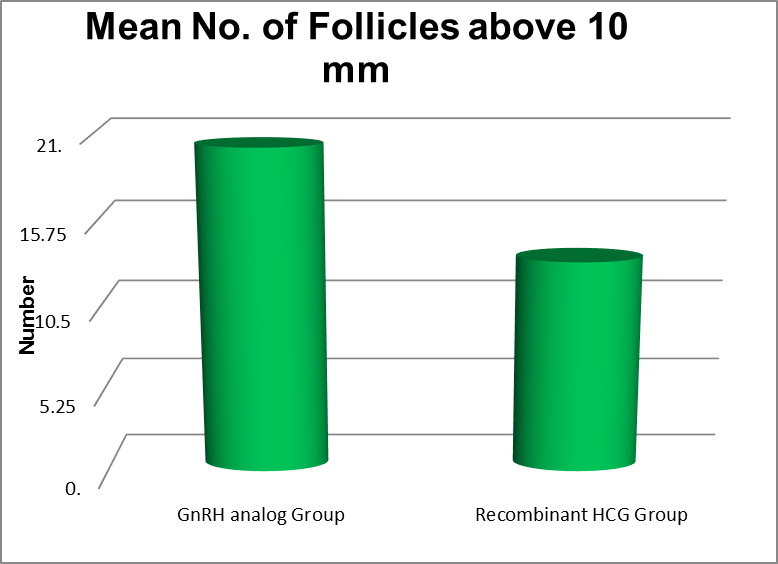
No. of Follicles above 10 mm |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
9 |
30 |
20.28 |
5.365 |
<0.001 |
|
Recombinant HCG Group |
8 |
25 |
13.33 |
4.782 |
|
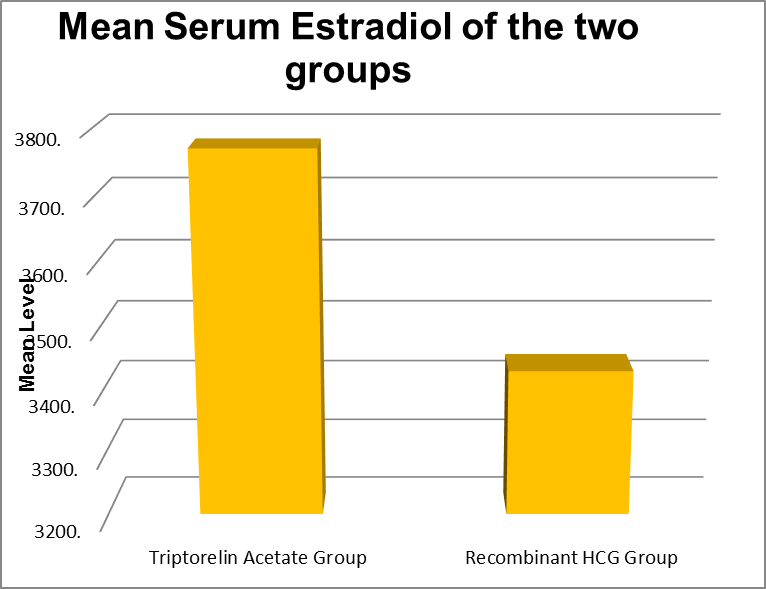
Serum Estradiol on the day of trigger (pg/ ml) |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
2408 |
4981 |
3773.02 |
597.461 |
0.326 |
|
Recombinant HCG Group |
3002 |
4496 |
3431.59 |
350.918 |
|
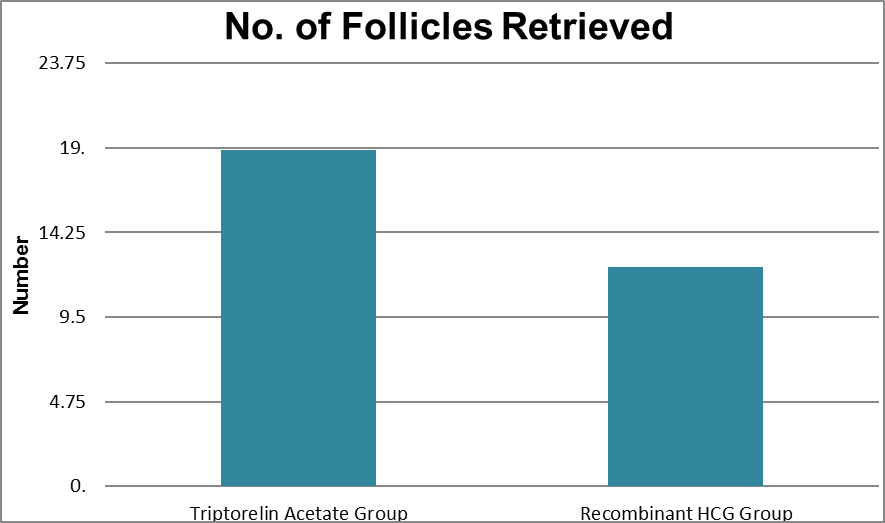
No.of follicles Retreived |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
8 |
28 |
18.85 |
5.249 |
<0.001 |
|
Recombinant HCG Group |
5 |
22 |
12.33 |
3.972 |
|
Proportion of Follicles that were aspirated (%) |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
.00 |
100.00 |
89.51 |
19.01 |
0.281 |
|
Recombinant HCG Group |
58.00 |
100.00 |
92.82 |
11.79 |
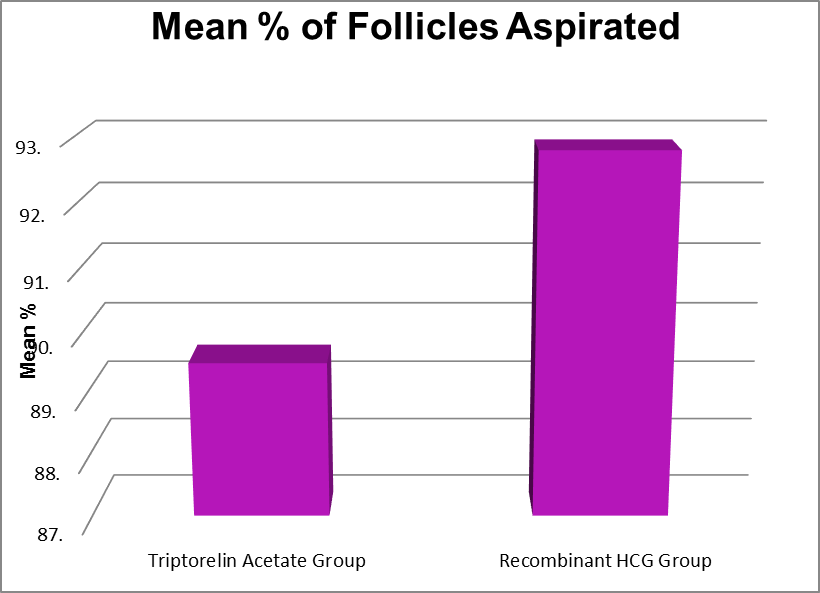
|
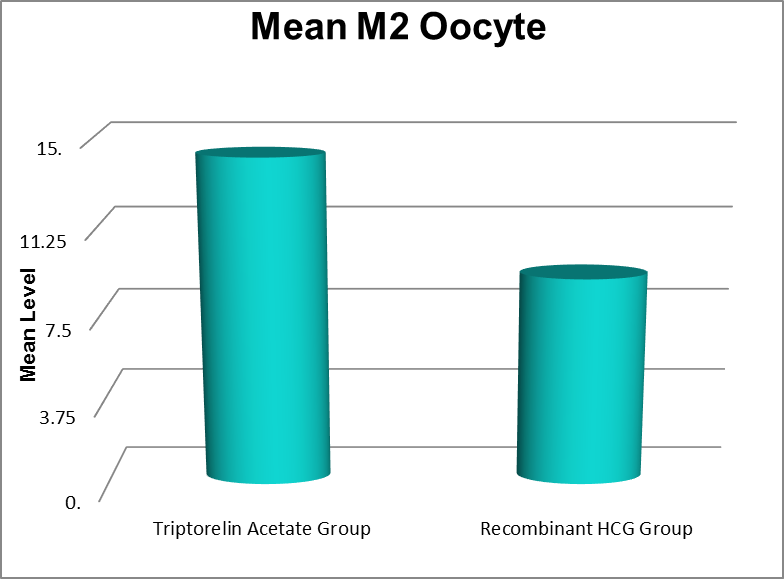
M2 Oocytes |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
6 |
25 |
14.26 |
4.776 |
<0.001 |
|
Recombinant HCG Group |
1 |
18 |
9.11 |
3.884 |
|
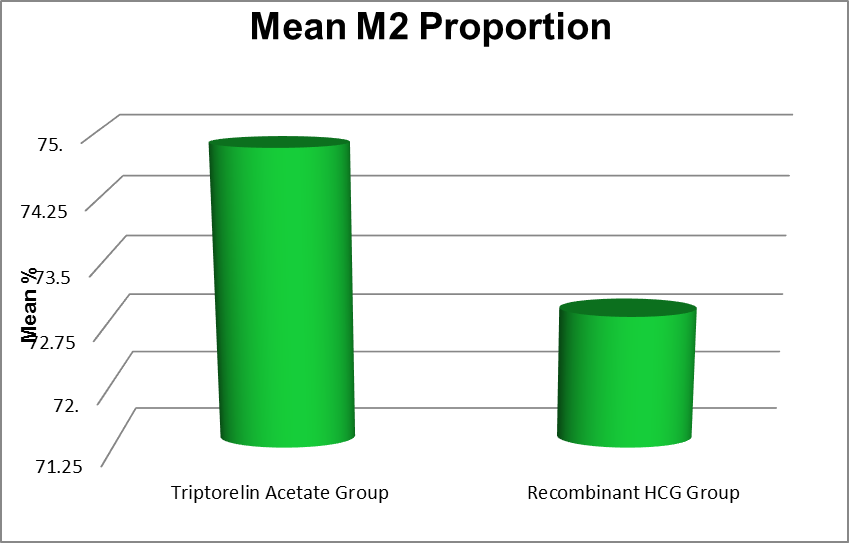
M2 Proportion ( %) |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
18.10 |
100.00 |
74.84 |
15.19 |
0.537 |
|
Recombinant HCG Group |
16.60 |
100.00 |
72.86 |
17.82 |
|
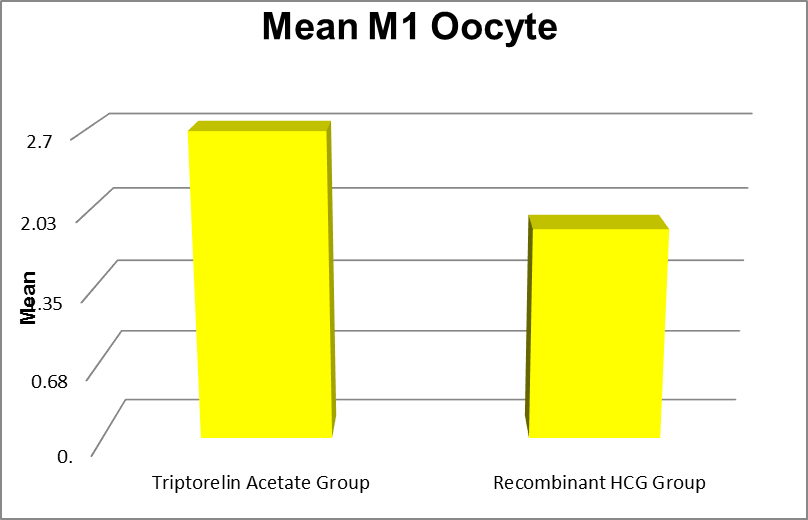
M1 oocytes |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
0 |
9 |
2.70 |
1.98 |
0.015 |
|
Recombinant HCG Group |
0 |
9 |
1.87 |
1.42 |
|
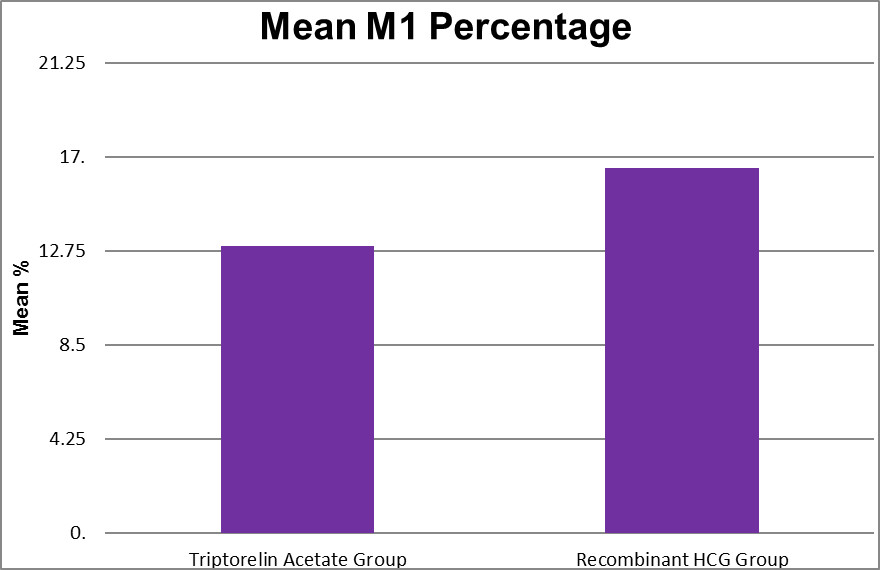
M1 Percentage |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
.00 |
36.20 |
12.96 |
9.57 |
0.155 |
|
Recombinant HCG Group |
.00 |
90.00 |
16.49 |
15.17 |
|
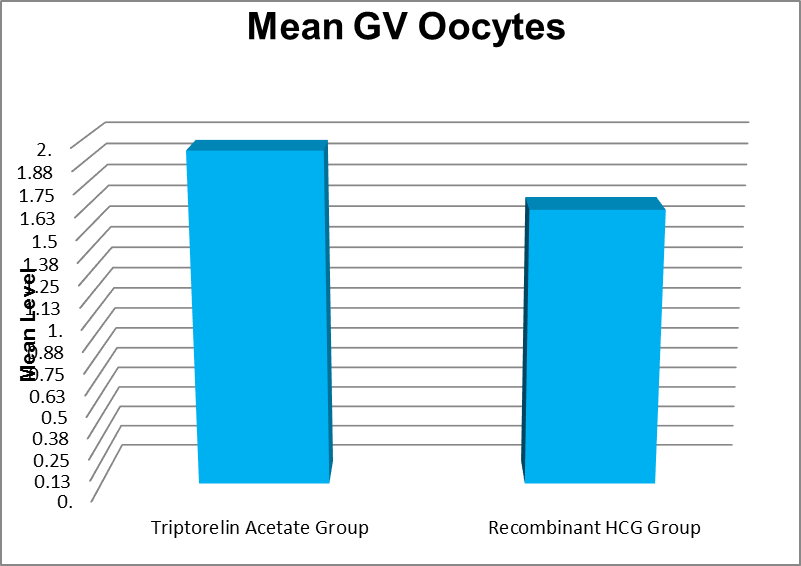
GV Oocytes |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
0 |
6 |
1.94 |
1.46 |
0.166 |
|
Recombinant HCG Group |
0 |
4 |
1.61 |
.96 |
|
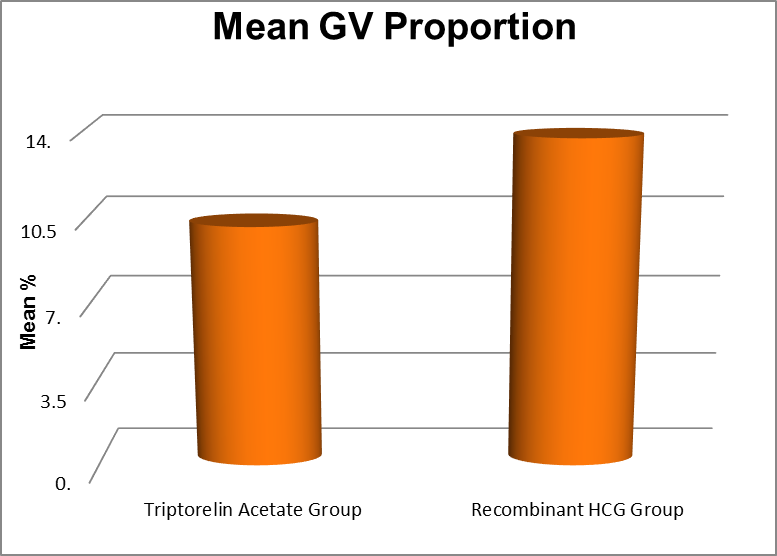
Proportion GV ( %) |
Minimum |
Maximum |
Mean |
Std. Deviation |
P value |
|
Triptorelin Acetate Group |
.00 |
31.25 |
10.17 |
7.29 |
0.015 |
|
Recombinant HCG Group |
.00 |
36.00 |
13.76 |
7.71 |
Secondary Outcom
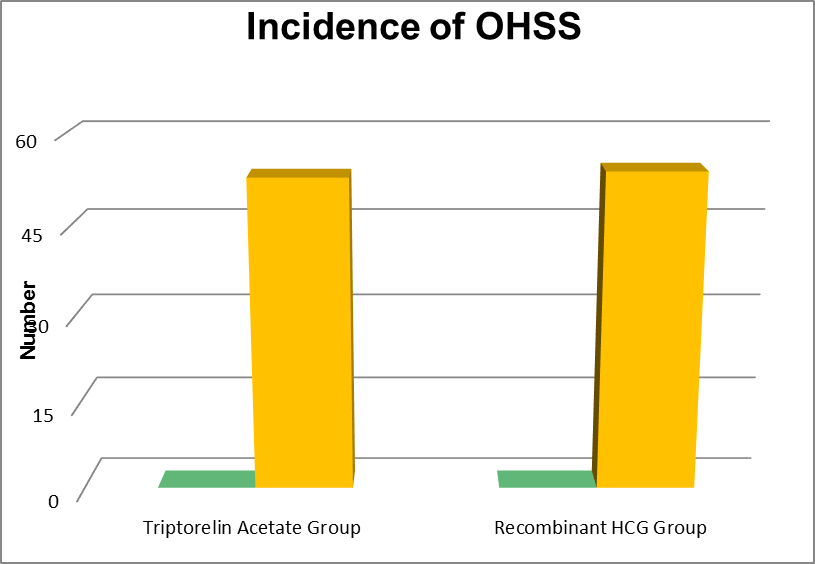
|
Group |
Incidence of OHSS |
Total Participants |
|
Triptorelin Acetate Group |
0 |
53 |
|
Recombinant HCG Group |
0 |
54 |
The groups were analysed by age AMH BMI AFC and serum estradiol value on the day of trigger .
In Recombinant HCG trigger group 7 patients were 21-25 years, 17 patients were 26-30 years and 27 patients were 31 to 35 years . And in Triptorelin acetate trigger group 9 patients were 21-25 years 24 patients were 26-30 years and 20 patients were 31-35 years. The mean value was 30.24+|- 3.23 in the Recombinant HCG trigger group and 29.17 +|- 3.15 in the Triptorelin acetate trigger group. The P value was 0.0858.
The AMH value in the Recombinant HCG trigger group, 25 patients had AMH 1.6-2 ng/ml, 16 patients had 2.1-2.5 ng/ml, 5 patients had 2.6-3.0 ng/ml, 4 patients had 3.1-3.5 ng/ml and 2 patients had 3.5-4 ng/ ml. In the Triptorelin acetate trigger group, 9 patients had 1.6 to 2 ng/ ml, 6 patients had 2.1-2.5 ng/ml, 8 patients had 2.6 to 3.0 ng/ml 18 patients had 3.1 to 3.5 ng/ml and 13 patients had 3.6 to 4 ng/ml The mean value was 2.21+|- 0.60 in the Recombinant HCG trigger group and 3.02+|- 0.7 in Triptorelin acetate trigger group. The P value was < 0.0001.
24 patients had Body mass Index (BMI) of less than 24 and 30 patients had BMI more than or equal to 25 and less than 30 in the Recombinant HCG Trigger group and 12 patients had BMI less than 24 and 41 patients had BMI more than 25 but less than 30 in the Triptorelin acetate trigger group. and Mean value was 24.70 +|- 2.86 in Recombinant HCG trigger group and 26.52+|- 2.06 in the Triptorelin acetate trigger group. The P value was 0.0003.
32 patients had 8-15 antral follicular count AFC and 22 patients had 16-24 AFC in recombinant HCG group . And in the Triptorelin acetate trigger group 10 patients had AFC 8-15 and 43 patients had 16-24 AFC. The mean value was 15.20+|- 4.51 in the Recombinant HCG trigger group and 19.06+|- 4.44 in the Triptorelin acetate trigger group. The P value was <0.0001
30 patients had serum estradiol value 3001-3500 pg/mL, 21 patients had 3501-4000 pg/mL. 4
patients had 4001 to 4500 pg/mL and No one had 4501-5000 pg/mL in the Recombinant HCG trigger group. And in the Triptorelin acetate trigger group 22 patients had 3001 -3500 pg/mL, 15 patients had 3501-4000 pg/mL, 7 patients had 4001-4500 pg/mL and 9 patients had 4501 -5000 pg/mL. The mean value was 3427.24 +|- 344.26 in the Recombinant HCG trigger group and 3773.02+|- 591.81 in the Triptorelin acetate trigger group. The P value was 0.0003.
Discussion
Although all oocytes are exposed to the LH surge from the trigger to initiate final oocyte maturation at the same point in time, oocytes retrieved are not only at different stages of nuclear maturity, but probably also have different cytoplasmic maturity. Oocyte maturation has been defined as the period taken by an oocyte to progress from the first to the second meiotic arrest, which involves coordinated, but asynchronous nuclear and cytoplasmic maturational modifications 7, 8.
An appreciation of the endocrine and temporal requirements for oocyte maturation enables the
optimization of current IVF protocols and the development of novel approaches to induce oocyte maturation to improve both the safety and efficacy of IVF treatment. Though there are many studies comparing the effect of recombinant HCG and GnRH agonist , to the best of our knowledge, this is the only study comparing the effects of recombinant HCG and Triptorelin acetate specifically about the effects of Oocyte maturity rate.
In some studies, the use of GnRH-a in the final oocyte maturation has similar or better results compared to hCG trigger (Humaidan et al 2013, Reddy et al 2014) . Unlike hCG trigger, GnRH-a trigger stimulates FSH surge in addition to LH surge. FSH surge, in the mid-cycle, has a specific effect on oocyte maturation and leads to a further expansion of cumulus cells surrounding the oocyte and release of proteolytic enzymes involved in the process of ovulation(Richards et al). Lamb et al by adding a dose of FSH to the hCG trigger, showed better recovery of oocyte and higher fertilization rates in IVF compared with hCG trigger alone.
Another advantage of this method is more maturity of the nucleus and the resumption of meiosis and eventually increasing the number of Metaphase II oocytes (Anderson et al 1999). In addition, increased levels of LH following injection of hCG is slower than that following GnRH-a trigger (Shapiro et al)
The present prospective randomised study was conducted to assess the efficacy of two different triggers , Triptorelin acetate 0.2 mg administered subcutaneously and recombinant HCG 250mg administered subscutaneously, for triggering final oocyte maturation in antagonist co-treated ICSI freeze all cycles. The results show that patients triggered with Triptorelin acetate had more yield of mature metaphase 2 oocytes than the patients triggered with Recombinant HCG. This may be due the fact that administration of GnRH agonist results in endogenous rise in both LH and FSH levels from the pituitary gland owing to initial flare effect similar to that of natural cycle (Gonen et al., 1990).
Where as recombinant HCG causes only mimics LH activity. Moreover, GnRH agonist endorses oocyte maturation, reinitiating meiosis and cumulus’ expansion, and reporting high mature oocyte rates (Andersen et al., 2006; Humaidan et al., 2011).
The evidence from these studies clearly illustrates that routine COS can be modified to produce more in vivo matured oocytes which has been shown by our study and other studies to be critical to the development of competent embryos with high implantation potential.
Moreover oocyte maturity produced in vivo determine the fertilization potential and subsequent blastocyst quality in vitro.(Lin YC 2003)
Replacing recombinant HCG with a single bolus of GnRH agonist for final oocyte maturation prevents OHSS and permits the continuation of the treatment cycle. Several authors have studied the applicability of this regime regarding this issue (Emperaire and Ruffie, 1991; Itskovitz et al., 1991; Kol et al., 1996; Lewit et al., 1996; Babayof et al., 2006; Engmann et al., 2006, 2008). A shorter half-life of the endogenous LH surge and the subsequent pituitary suppression that leads to early luteolysis and a reduced luteal-phase steroidal concentration may explain the lower incidence of OHSS among patients submitted to GnRH agonist triggering (Lanzone et al., 1989; Beckers et al., 2003; Emperaire et al., 2004; Andersen et al., 2006). In the present study, none of the oocyte donors undergoing induction of final oocyte maturation developed OHSS while eight of the donors in the other group did. This finding is supported by previous reports in the literature.
Humaidan et al. (2005) retrieved significantly more MII oocytes after a GnRH agonist was used to trigger ovulation. The explanation for this result was that optimal oocyte maturation may have been achieved by a simultaneous and coordinated effect of a mid-cycle FSH and LH surge.The additional FSH surge induced is believed to promote the resumption of oocyte meiosis, LH receptor formation, cumulus expansion, and release of proteolytic enzymes involved in ovulation.[7,8. Eppig JJ.1979]
However, the use of GnRHa alone as a trigger results in a lower pregnancy rate and an extremely high early pregnancy loss rate due to a luteal phase insufficiency.[11. Kobilanski et al 2005] In our study all patients were for Frozen Embryo transfer .
Evidence suggests that GnRH agonist as a final oocyte maturation trigger in fresh autologous cycles is associated with a lower live birth rate, a lower ongoing pregnancy rate (pregnancy beyond 12 weeks) and a higher rate of early miscarriage (less than 12 weeks). GnRH agonist as an oocyte maturation trigger could be useful for women who choose to avoid fresh transfers (for whatever reason), women who donate oocytes to recipients or women who wish to freeze their eggs for later use in the context of fertility preservation.
Conclusion
Triptorelin acetate and recombinant HCG triggers are effective and safe method for final oocyte maturation.
Triptorelin acetate, may yield more number of Metapahse 2 oocytes than recombinant HCG trigger.
infrozen embryo transfer
More studies and researches might be needed to confirm our findings and improve our understanding.
GnRH agonist as a final oocyte maturation trigger in fresh autologous cycles is associated with a lower live birth rate, a lower ongoing pregnancy rate (pregnancy beyond 12 weeks) and a higher rate of early miscarriage (less than 12 weeks).
GnRH agonist as an oocyte maturation trigger could be useful for women who choose to avoid fresh transfers (for whatever reason), women who donate oocytes to recipients or women who wish to freeze their eggs for later use in the context of fertility preservation.
Certificate
This is to certify that this study titled Comparison of Oocyte Maturation in Recombinant Human Chorionic Gonadotrophin and Triptorelin Acetate Trigger ; A randomised prospective study is a bonafide research work carried out by Dr. Priya Senthil Reg no 111992004 in the Department of Reproductive Medicine under our guidance and being submitted in partial fulfillment for the degree of
Fellowship in Reproductive Medicine in January 2020.
Acknowledgment
It is with the sense of immense respect, I express my sincere thanks to Dr Saravanan Lakshmanan and my Guide Dr Mahalakshmi Saravanan, my Teacher, my Mentor for their guidance and encouragement. Without their support this would not have been possible.
I would like to extend my sincere thanks to my Co Guide Dr Nithi Sharma , Professor Department of OBG Saveetha Medical College and Hospital, for her extreme kindness and guidance throughout the fellowship course. Very knowledgable yet humble person. Blessed to know you Mam .
I would like to thank our Chancellor Dr. N.M Veeraiyan and Director Dr.Saveetha Rajesh, Saveetha Medical College and Hospital for the high curricular standard they keep up.I also would like to thank Dr Jayashree Professor HOD, Department of Obstetrics and Gyneacology,, Saveetha Medical College and Dr Damodaran , Dean Saveetha Medical College for their support .
I also would like to express my regards to Dr.Avanthi, Dr Sindhuja , my Colleagues and Staff who helped me in the data collection and thesis work. And of course, all those 100 participants of this study, played an integral role, Without them agreeing, this study would not have been possible. I thank all of them from the bottom of my heart.
I also would like to thank my Husband Mr. Senthil and my sons Surya and Shatriya for all the happiness they have brought in my life and for their understanding and adjustment for my busy schedule . And I thank my Mother in law for taking care of my children and Dr Adityan my Nephew for his support.
And finally, I thank the Supremo, The Almighty, for making me what I am today .