
Vishal Chandra1, Haldher Dubey1, Pramod Yadav 1,2*
1School of Life Sciences and Biotechnology, Chhatrapati Shahu Ji Maharaj University Kanpur, Uttar Pradesh, India.
2Amity Institute of Neuropsychology and Neurosciences, Amity University Uttar Pradesh, India.
*Corresponding author: Pramod Yadav, Department of AFAF, Amity University Uttar Pradesh, Noida Campus, 201313, India.
Received Date: December 04, 2023
Accepted Date: December 14, 2023
Published Date: December 20, 2023
Citation: Yadav P, Chandra V, Dubey H, (2023). “Purifying Bacillus Subtilis Protease: A Step Closer to Commercialization”. Molecular Biology and Biochemistry, 1(1); DOI: /MBB/004.
Copyright: © 2023 Pramod Yadav, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This study reports the isolation and characterization of a novel protease-producing strain of Bacillus subtilis. The isolate, designated as GS-P40, exhibited the highest protease activity among six proteolytic bacterial isolates screened on skim milk agar plates. Morphological and biochemical features confirmed the identity of GS-P40 as B. subtilis. Protease production and activity were optimal at pH 7.4, temperature 60°C, and fermentation time 48 h. The crude protease concentration was 0.14 μM with a specific activity of 0.0233 U/ml. The crude protease also improved the washing performance of a commercial detergent, indicating its potential as a biotechnological agent. This paper presents the isolation and characterization of a novel protease-producing B. subtilis strain and its potential application in the detergent industry.
protease; enzyme isolation; enzyme characterization; fermentation; biotechnology
Introduction:
The isolation of pure cultures of microorganisms from environmental or host-associated samples is essential for identification purposes. This technique has a long history in bacteriology and parasitology and later in virology, with continuous improvements in automation, technology, speed, and accuracy [1,2]. A gram-positive, motile, and endospore-forming microorganism, Bacillus subtilis is widely distributed in nature. It synthesizes and releases different types of antibiotics, including polymyxin, difficidin, subtilin, and mycobacillin, as part of its sporulation process. The biosynthesis and secretion of these antibiotics are mediated by five single peptidase genes in B. subtilis [3]. Bacillus spp. are industrially relevant for the production of alkaline proteases, which are enzymes that hydrolyse peptide bonds in proteins under alkaline conditions. Among the commercially available alkaline proteases, those derived from Bacillus spp., such as B. cereus, B. sterothemophilus, mojavensis, and B. subtilis, are widely used [3,4]. Alkaline proteases account for a large share (59%) of the global industrial enzyme market, which was valued at over $5.9 billion in 2023 [5]. These enzymes have applications in various sectors, such as detergents, leather, food, and pharmaceuticals. Microorganisms are preferred sources of proteases due to their fast growth rate, low cultivation space requirement, and genetic manipulability, which enable the production of tailor-made enzymes for specific purposes [5]. To identify bacterial isolates, 16S rDNA sequence analysis was performed. DNA was extracted from single colonies by alkaline lysis and stored at -20°C until further use. The 16S rDNA region was amplified and sequenced using specific primers, and the sequences were edited using Chromas LITE (version 2.01) and compared with GenBank entries using BLAST to determine the closest matches. A neighbor-joining phylogenetic tree was constructed based on the 16S rDNA sequences using Clustal W. Polymerase chain reaction (PCR) is a method for in vitro DNA amplification that uses two oligonucleotide primers that anneal to opposite strands of the target DNA fragment and serve as templates for DNA synthesis by a polymerase. This study isolated and characterized a protease-producing B. subtilis strain from soil samples using biochemical and molecular methods. It also evaluated the enzymatic activity and stability of the protease under different conditions and compared it with commercially available proteases. The results suggest that the isolated B. subtilis strain is a potential source of alkaline protease for industrial applications.
Review of literature:
Novel antimicrobial agents are needed to combat emerging antibiotic-resistant pathogens in human, animal, and plant diseases. Marine bacteria are a potential source of such compounds, as they produce antibacterial substances. Antimicrobial substances from microorganisms in sea water have been identified, such as bacitracin from Vibrio harveyi, named harveyicin. Bacteria exhibit a remarkable defense mechanism by producing bacteriocins, ribosomally synthesized peptides with bactericidal activity [6]. Various gram-negative and gram-positive bacteria produce bacteriocins, with lactic acid bacteria (LAB) being the most promising producers [7]. LAB bacteriocins are generally recognized as safe (GRAS) and exhibit antibacterial activity against food-borne pathogens and spoilage bacteria, making them attractive for food biopreservation [8,9]. The main classification scheme for LAB bacteriocins is based on the mode of action and activity spectrum. Lantibiotics are a group of class I bacteriocins that are characterized by their small size, membrane activity, heat stability, and presence of uncommon thioether amino acids. Class II bacteriocins share the same features except for the modification of their amino acids, and they are further classified into three subclasses based on their structure and function [10]: Class IIa, Class IIb, and Class IIc. Class IIa bacteriocins are characterized by a conserved motif at their N-terminal ends and exhibit activity against Listeria. Class IIb bacteriocins are composed of two distinct peptides that need to interact for optimal activity. Class IIc bacteriocins are secreted by the sec-system and depend on it for their function [10]. Class III bacteriocins are large proteins that are heat-sensitive and exported by the bacterial preprotein translocase (sec-pathway) [11]. Class IV includes circular bacteriocins that are covalently linked head to tail [9,12]. LAB bacteriocins are currently authorized for use as food preservatives, with nisin being the most well-known and studied [9]. Bacteriocins have been isolated from various Bacillus species. A bacteriocin-like inhibitory substance (BLIS) producer, Bacillus licheniformis exhibits a wide range of antimicrobial activity against harmful and deteriorating bacteria. Bacillus subtilis strains produce antibacterial and antifungal compounds such as subtilin and ericins. Bacillus cereus produces cereins active against other B. cereus strains [11,13]. Bacillus amyloliquefaciens produces BLIS active against E. coli and Clostridium perfringens. Other Bacillus species such as B. polymyxa, B. megaterium, B. mycoides, and B. pumilus also produce bacteriocins. In addition to bacteria, some of the BLIS synthesized by Bacillus species exhibit antimicrobial activity against fungi. B. licheniformis, B. brevis, and B. amyloliquefaciens produce BLIS with broad-spectrum antagonistic activity against various bacteria and fungi [13].
Table 1: Bacillus species and their bacteriocin production.
|
Species |
Source |
Antimicrobial Activity |
Bacteriocin |
References |
|
Bacillus subtilis |
Soil, water |
Yes |
Subtilin and Ericins |
[14] |
|
Bacillus licheniformis |
Soil, feathers, food |
Yes |
Bacteriocin like inhibitory substances |
[15] |
|
Bacillus amyloliquefaciens |
Soil, water, plants |
Yes |
Bacteriocin like inhibitory substances |
[16] |
|
Bacillus pumilus |
Soil, water |
Yes |
Bacteriocin |
[11] |
|
Paenibacillus sp. |
Soil, water |
Yes |
Bacteriocin |
[9] |
Materials and methods:
Soil samples were collected from the garden of the IBSBT department at Chatrapati Sahu Ji Maharaj University, Kanpur, India, at a depth of 4-6 cm and stored in sterile plastic bags with date and time labels. The aim of this study was to isolate and identify a bacterial strain capable of hydrolyzing casein, the main protein in milk. Casein hydrolysis is an important process in the dairy industry, as it affects the quality and texture of cheese and other dairy products. The following methods were used to isolate and identify the bacterial strain [17,18]. Serial dilutions (10-1 to 10-6) of the soil samples were prepared and spread on skim milk agar plates (1%). After incubating the plates at 37°C for 48 hours, the hydrolysis zone around each colony was measured [19]. The colony with the largest zone of hydrolysis was selected and subcultured on nutrient agar plates for further identification. Morphological and biochemical tests were performed to characterize the bacterial isolate. Additionally, molecular identification was carried out using two methods: 16S rRNA gene sequencing and polymerase chain reaction (PCR).
Morphological and biochemical tests
Gram staining test:
The morphological features of the bacterial isolate were observed using a light microscope after Gram staining. Gram staining is used to distinguishes bacteria based on their cell wall configuration. Gram (+ve) bacteria possess a thick peptidoglycan layer that holds the crystal violet (primary stain), whereas gram (-ve) bacteria have a thin layer of peptidoglycan [20]. A heat-fixed smear of the bacterial culture was prepared on a glass slide. The slides were stained with primary stain for one minute, washed with tap water, treated with primary stain fixation (Gram’s iodine) for one minute to form a complex with the primary stain, decolorized with ethyl alcohol for about three seconds, again washed with tap water, stain with counterstained (safranin) for one minute, and observed under oil light microscope [21,22].
Catalase test:
The bacterial isolate was subjected to catalase and gelatine hydrolysis tests. The catalase test detects the presence of catalase enzyme in bacteria, which decomposes H2O2 into H2O and O2. This test was performed using the following protocol: a loopful of the bacterial isolate was smeared on a glass slide and overlaid with 3% hydrogen peroxide solution. The formation of oxygen bubbles within one minute specified a (+ve) catalase reaction, while the lack of bubbles showed a (-ve) catalase reaction [23,24].
Gelatine hydrolysis test:
The gelatin hydrolysis test evaluates the production of gelatinase, an enzyme that cleaves gelatin into amino acids, by bacteria. Gelatine is a protein derived from collagen, which is found in animal connective tissues. The procedure for gelatine hydrolysis test was as follows: the gelatine medium (consisting of 0.5% peptone, 2% gelatine, 0.3% beef extract, 0.5% sodium chloride, and pH 7.2) was melted in a water bath, cooled to 45-50°C, and dispensed into four sterile test tubes, allowing it to solidify. The nutrient gelatine tubes were then labelled with the names of the bacterial isolates to be inoculated [25]. A stab inoculation was performed from each culture into an appropriately labelled deep tube of nutrient gelatine, while an uninoculated deep tube served as the control. The inoculated and control tubes were incubated at 37°C for 4-7 days. Then, the tubes were refrigerated at 4°C for 15 minutes to induce gelatin solidification. The tubes were then observed for liquefaction or solidification of gelatine [25,26].
MRVP test:
The MRVP (methyl red and Voges-Proskauer) test is a differential test that distinguishes enteric bacteria based on their metabolic products from glucose fermentation. The test comprises two components: the MR and the VP tests. The MR test finds mixed acid fermentation, which reduces the medium's pH. The VP test detects acetoin production, a precursor of 2,3-butanediol [27]. Typically, bacteria that produce mixed acids are MR positive and VP negative (MR +ve, VP –ve), while bacteria that produce 2,3-butanediol are MR negative and VP positive (MR –ve, VP +ve). To perform the MRVP test, 5 ml of MRVP broth (containing 0.7% peptone, 0.5% glucose, and 0.5% potassium phosphate) was inoculated with Bacillus subtilis and sterilized by autoclaving at 121°C for 15 minutes. A control tube with uninoculated broth was also prepared. The inoculated and control tubes were incubated at 35°C for 48 hours. After incubation, the tubes were divided into two equal parts for the MR and VP tests. For the MR test, five drops of methyl red indicator (pH 4.4) were added to one part of the inoculated tube and the control tube [28]. The tubes were gently agitated and the color change was noted. A red color signified a positive MR test (pH ≤ 4.4), whereas a yellow or orange color signified a negative MR test (pH > 6). For the VP test, 12 drops of VP reagents A (5% α-naphthol) and B (40% KOH) were added to the other part of the inoculated tube and the control tube. The tubes were gently shaken for 30 seconds with the caps off to aerate the medium. The tubes were then left undisturbed for 15-30 minutes and observed for color change. A red or pink color in the upper part of the medium signified a positive VP test, whereas no color change or a copper color signified a negative VP test [25].
Citrate utilization test:
The citrate utilization test assesses the bacterial capacity to consume citrate as only C2 source and ammonium salts (such ammonium chloride) as only N2 source. This ability depends on the presence of citrate permease, an enzyme that transports citrate into the cell, and citrase, an enzyme that cleaves citrate into oxaloacetate and acetate [29]. Oxaloacetate is then converted to pyruvate and CO_2 by various enzymes, while acetate is converted to acetyl-CoA and then to CO_2 and H_2O by the tricarboxylic acid cycle. The utilization of citrate and ammonium salts results in the production of ammonia and sodium carbonate, which raise the pH of the medium. The citrate utilization test is performed by streaking the bacterial isolate on Simmons' citrate agar slants [30]. Simmons' citrate agar comprises sodium citrate, the only carbon source, ammonium dihydrogen phosphate, the only nitrogen source, sodium chloride, magnesium sulfate, agar, and bromothymol blue as a pH indicator. The medium has an initial pH of 6.9 and is green in color. The slants were incubated at 37°C for 4-7 days. Growth and a blue color change of the medium (pH > 7.6) signified a positive citrate utilization test. A negative citrate utilization test is indicated by no growth and no color change of the medium (pH below 7.6) [31].
Molecular identification:
To confirm the identity of the bacterial isolate, molecular methods were employed, including 16S rRNA gene sequencing and polymerase chain reaction (PCR). These methods rely on the amplification and analysis of specific DNA regions that are conserved or variable among different bacterial taxa.
Genomic DNA extraction:
The bacterial isolate was cultured in nutrient broth at room temperature overnight with shaking. The cells were centrifuged (RPM 10000 and three minutes) and supernatants werre discorded while pallets were resuspended in 500 µl of 1X TE buffer which are consist of 1 mM EDTA, 10 mM Tris-HCl, pH 8.0). To lyse the cells, 50 µl SDS (10%) and 5 µl proteinase K in concentration of (20 mg/ml) were put in and mixed thoroughly. The lysate was incubated at 37°C for 2 h to digest RNase and any residual RNA [32]. The genomic DNA was extracted with chloroform:isoamyl alcohol (24:1) in a 1:1 ratio and centrifuged (RPM 10000 and five minutes) followed by vortexing. The DNA-containing supernatants were moved to a new centrifuge tube and the extraction was repeated until no interphase was observed. By putting the sodium acetate (3M and pH 5.2) at 1/10 volume, the DNA was precipitated and isopropanol at 0.5 volume, followed by gentle inversion until a white precipitate formed. Then, DNA was centrifuged (RPM 10000 and five minutes) and the pelleted was cleaned with ethanol (70%). Further, the DNA pellets were air-dried and re-dissolved in 30-40 µl of 1X TE buffer for further analysis [32].
PCR amplification:
PCR is a technique that allows the exponential amplification of a specific DNA fragment in vitro using primers, nucleotides, and a thermostable DNA polymerase. The primers are short synthetic oligonucleotides that anneal to the complementary sequences flanking the target region on the template DNA. In this study, we used PCR to amplify a ~1500 bp fragment of the 16S rRNA gene, which is a highly conserved gene that encodes a component of the bacterial ribosome. The 16S rRNA gene comprises both variable and conserved regions that are useful for phylogenetic analysis and identification of bacteria at different taxonomic levels [33]. We used the universal primers 27F (5'-AGAGTTTGATCMTGGCTCAG-3') and 1492R (5'-TACGGYTACCTTGTTACGACTT-3'), that direct the conserved regions at the ends of the gene. The PCR reaction mixture consisted of 2 µl of target DNA (~50 ng), 0.6 µl primers (10 pmol/µl), 0.6 µl of Taq DNA polymerase (5 U/µl), 0.5 µl of dNTPs (10 mM each), 2 µl of 10X PCR buffer with MgCl2. PCR buffer consists of Tris-HCl (200 mM pH 8.4), KCl (500 mM), and MgCl2 (15 mM), and 15 µl of nuclease-free water, for a total volume of 20 µl. We placed tube containing all reaction in PCR machine by applying the programs [33,34].
Agarose gel electrophoresis:
Agarose gel electrophoresis is a technique that resolves DNA molecules according to their size and charge. Agarose is a seaweed-derived polysaccharide that forms a gel matrix upon dissolution in a buffer and cooling. The gel has pores that allow the movement of DNA molecules under the influence of an electric field. The phosphate backbone confers a negative charge to the DNA molecules, which migrate toward the anode during electrophoresis [35]. The smaller DNA molecules move faster and farther than the larger ones, resulting in a size-based separation. We visualized the DNA molecules by staining the gel with ethidium bromide (EtBr), a fluorescent intercalating agent that emits orange light upon UV irradiation [36]. A molecular weight marker, which is a mixture of DNA fragments of known sizes, is run alongside the samples to estimate the size of the PCR products. We used agarose gel electrophoresis to verify the quality and quantity of the genomic DNA and the PCR products. We prepared an 0.8% agarose gel for 100 ml in 1X TAE buffer which consists of 1 mM EDTA, 40 mM Tris-acetate, and pH 8.0. We placed the gel in an electrophoresis tank and filled this tank with 1X TAE buffer. We mixed 5 µl of genomic DNA with 1 µl of 6X loading dye (0.25% xylene cyanol, 30% glycerol, 0.25% bromophenol blue) and load up into the wells. We also loaded 5 µl of 1 kb DNA ladder (a molecular weight marker) into a separate well. We performed electrophoresis at 100 V for about an hour or until the dye front reached the end of the gel. We visualized and photographed the gel under UV light using a gel documentation system [35–37].
Result:
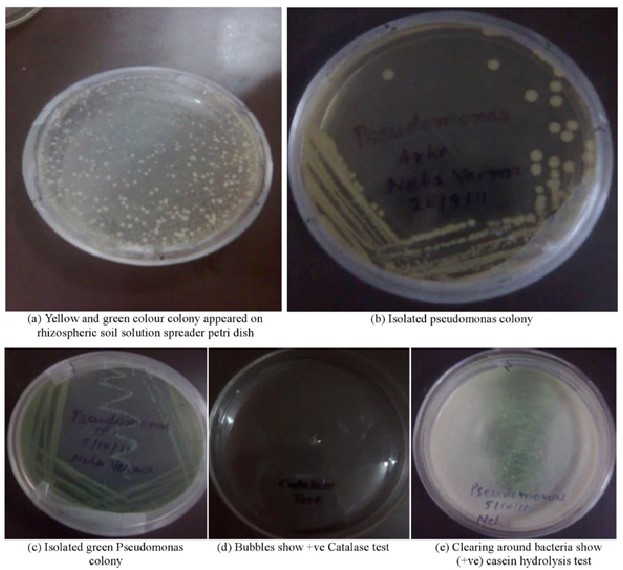
Figure 1: Petri dish results of various assays (a) Rhizospheric soil spreading (b and c) Pseudomonas colony isolation (d) Catalase activity (e) Casein hydrolysis.
We obtained pure bacterial colonies by serial dilution and plating of the soil samples on nutrient agar. The colonies were then streaked on King's medium B agar plates, which is a selective medium for Pseudomonas species. Pseudomonas species produce pigments that give the colonies a yellow or green color on King's medium B agar plates. The colonies that did not show any pigmentation were selected for further biochemical tests. The biochemical tests performed on the selected colonies included catalase test, gelatin hydrolysis test, indole production test, MR-VP test, citrate utilization test, and casein hydrolysis test. The catalase test detects the catalase enzyme, which catalyzes the decomposition of H2O2 into H2O and O2. Oxygen bubbles form when H2O2 is added to a positive catalase culture. The gelatin hydrolysis test assesses the ability of bacteria to produce gelatinase enzyme, which hydrolyzes gelatin into amino acids. A positive gelatin hydrolysis test is indicated by the liquefaction of the gelatin medium after refrigeration. The indole production test assesses the bacterial ability to produce indole from tryptophan by tryptophanase. A positive indole production test is indicated by a red color in the reagent layer when Kovac's reagent is added to the bacterial culture. The MR-VP test distinguishes bacteria based on their glucose fermentation products. The MR test detects mixed acids, which acidify the medium, while the VP test detects acetoin, a 2,3-butanediol precursor. After adding methyl red in a red broth give a positive MR test, while after adding a VP reagent (A and B) in a red or pink broth gave a positive VP test. A positive citrate utilization test is indicated by growth on Simmons' citrate agar slants and a blue color change of the medium due to alkaline pH. The casein hydrolysis test evaluates the ability of bacteria to produce caseinase enzyme, which hydrolyzes casein, the main protein in milk. A positive casein hydrolysis test is indicated by a clear zone around the bacterial growth on skim milk agar plates.
Table 2: Summary of biochemical tests.
The molecular tests confirmed the identity of the isolated strain as B. subtilis based on the 16S rRNA gene sequence. The primers ((Bsub5F and Bsub3R) targeting a 595-bp DNA fragment, are used in this study were specific for the ‘Bacillus subtilis group’, which includes closely related species such as B. licheniformis, B. amyloliquefaciens, B. pumilus, and B. atrophaeus. These species share high similarity in their 16S rRNA gene sequences and are difficult to distinguish by conventional methods. Therefore, PCR and sequencing were necessary to verify the specificity of the isolated strain.
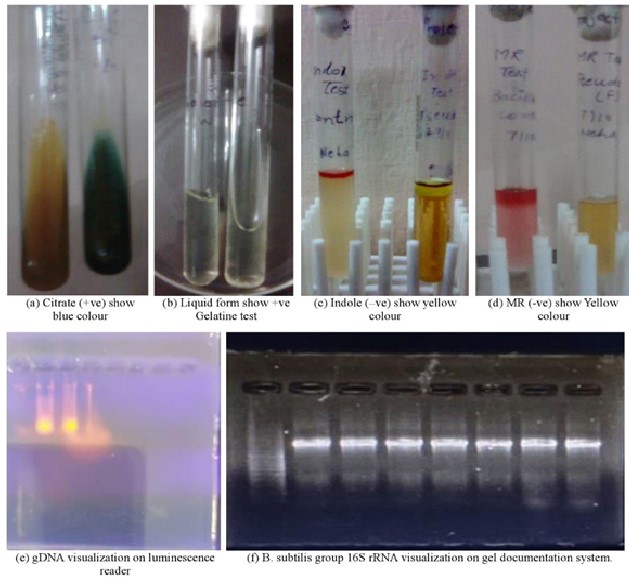
Figure 2: Assays for different biochemical and molecular characteristics (a) Citrate utilization (b) Gelatinee hydrolysis (c) Indole production (d) Methyl red and Voges-Proskauer reactions (e) Genomic DNA detection on luminescence reader (f) B. subtilis group 16S rRNA detection on gel documentation system.
Discussion:
The present study aimed to isolate and characterize a novel protease-producing Bacillus subtilis strain from soil samples collected at Kanpur University Campus, India. Among the 142 bacterial isolates screened for protease activity on skim milk agar plates, the isolate GS-P40 exhibited the highest protease activity and was selected for further analysis. Morphological and biochemical tests confirmed the identity of GS-P40 as Bacillus subtilis, a well-known producer of extracellular proteases [38,39]. The optimal conditions for protease production and activity by GS-P40 were determined to be pH 7.4, 60°C, and a fermentation time of 48 hours. The crude protease concentration and specific activity were estimated to be 0.14 μM and 0.0233 U/ml, respectively, using a tyrosine standard curve. The crude protease also showed the ability to enhance the washing performance of a commercial detergent, indicating its potential application in the detergent industry. Proteases are widely used in various industrial sectors, such as leather, food, and pharmaceutical industries, due to their catalytic properties and biodegradability [40]. Alkaline proteases, in particular, have high demand in the detergent industry, as they can function under alkaline conditions and remove proteinaceous stains effectively [41,42]. The novel protease-producing B. subtilis strain isolated in this study has significance for the production of alkaline proteases, as it can grow and secrete proteases under alkaline conditions. Moreover, B. subtilis is an advantageous source of proteases, as it has a fast growth rate, low cultivation space requirements, and genetic manipulability, enabling the production of customized enzymes for specific purposes [43,44]. To determine the exact taxonomic position of the isolated B. subtilis strain, 16S rDNA sequence analysis was performed using PCR primers Bsub5F and Bsub3R, which target a 595-bp DNA fragment of the ‘B. subtilis group’ 16S rRNA. The results showed high specificity for B. subtilis and its closely related species within the ‘Bacillus subtilis group’, such as B. licheniformis, B. amyloliquefaciens, B. pumilus, and B. atrophaeus [38,39,42,45]. These species are known to produce various types of proteases with different properties and applications, highlighting the potential relevance of the isolated strain for industrial enzyme production. However, the direct detection of B. subtilis from wastewater was difficult, possibly due to competition from partially matching 16S rRNA molecules or low abundance of the target strain in the environmental samples. The similar results are also reported by other studies on protease production by B. subtilis [1,46]. This limitation suggests the need for further optimization of detection methods and purification techniques to enable the reliable detection of B. subtilis in diverse environmental samples. The study provides valuable insights into the isolation, characterization, and potential industrial applications of a novel protease-producing B. subtilis strain. The favorable enzymatic properties of the crude protease, such as activity and stability under specific conditions, indicate the strain’s suitability for future biotechnological endeavors, including enzyme production for industrial purposes.
Conclusion:
In conclusion, this study observed that protease production by the isolated B. subtilis strain was influenced by several factors, such as pH, temperature, and fermentation time. The optimal conditions for protease production were pH 7.4, temperature 60°C, and fermentation time 48 h, which resulted in a crude protease concentration of 0.14 μM with a specific activity of 0.0233 U/ml. PCR and 16S rRNA sequencing verified the specificity of the isolated strain within the ‘Bacillus subtilis group.’ The protease produced by the isolated B. subtilis strain was alkaline and thermotolerant, which are desirable characteristics for industrial applications. The crude protease improved the washing performance of a commercial detergent, suggesting its biotechnological potential as an alkaline protease source for industrial applications.
Abbreviation:
|
DW |
- |
Distilled water |
|
RT |
- |
Room Temperature |
|
M |
- |
Molar Solution |
|
N |
- |
Normal Solution |
|
SDS |
- |
Sodium dodecyl Sulfate |
|
EDTA |
- |
Ethylene Diamine Tetra Acetate |
|
CBB |
- |
Commassie Brilliant Blue |
|
BPB |
- |
Bromophenol Blue |
|
TAE |
- |
Tri’s acetate EDTA |
|
NA |
- |
Nutrient agar |
|
LB |
- |
Luria Broth |
|
TE |
- |
Tris EDTA |
|
EtBr |
- |
Ethidium Bromide |
|
UV |
- |
Ultra violet |
|
OD |
- |
Optical Density |
|
PCR |
- |
Polymerase chain reaction |
|
RPM |
- |
Rotation per minute |
|
Psi |
- |
Pound per square inch |
|
dNTPs |
- |
Deoxy nucleotide triphosphate |
Competing interests: The authors report no conflicts of interest.
Author 1 declared no conflict of interest.
Author 2 declared no conflict of interest.
Author 3 declared no conflict of interest.
Funding: This research was unfunded by any public, commercial, or not-for-profit agencies.
Ethical Approval and Consent to Participate: Not applicable.
Guarantor: The article’s full responsibility lies with PY, who is the corresponding author and the third author in the list.
Authors' contributions: PY and HD were responsible for manuscript conceptualization, writing - original draft, ethical approvals, consent, and sample collection. VC participated in writing - review & editing.
Acknowledgements: The authors thank the Institute of Biosciences and Biotechnology, Chhatrapati Shahu Ji Maharaj University, Kanpur - 208024, India for providing the laboratory facilities.
Availability of data and materials: Data and materials are available upon request.
Consent for publication: All authors consented to manuscript publication.