
Gemechis Tuke1, Adulhalik Workicho2, Guta Kune2, Alqeer Aliyo3*, Miesa Gelchu1
1School of Public Health, Institute of Health, Bule Hora University, Bule Hora, Ethiopia
2School of Public Health, Institute of Health, Jimma University, Jimma, Ethiopia
3Medical Laboratory Science Department, Institute of Health, Bule Hora University, Bule Hora, Ethiopia
*Corresponding author: Alqeer Aliyo, Assistant professor. Medical Laboratory Science Department, Institute of Health, Bule Hora University, Bule Hora, Ethiopia.
Received Date: November 18, 2023
Accepted Date: Novemer 24, 2023
Published Date: December 06, 2023
Citation : Aliyo A, Tuke G, Workicho A, Kune G, Gelchu m, (2023).“Incidence rate and predictors of switching to second-line Antiretroviral Therapy among outpatient Adults with HIV at Adola and Negele General Hospital in Guji Zone, South Ethiopia”. Molecular Biology and Biochemistry, 1(1); DOI: /MBB/002.
Copyright: © 2023 Alqeer Aliyo, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: The emergence of drug resistance is of great concern as it leads to treatment failure. In Ethiopia, data on the causes and predictors of switching ART drug regimens between non-routine viral load monitoring settings are limited, and the need for secondary ART regimens is unclear.
Objective: This study aimed to determine the incidence and predictors of switching to secondary ART in HIV-positive adult outpatients at Adola and Negele General Hospital, Gujii zone, Southern Ethiopia, in 2021.
Methods: An institutional retrospective cohort research was carried out from June 2010 to June 2020. Data came from patient records that were chosen using simple random selection. EPI-Data version 4.6 was used to enter the data, and STATA version 15 was used to analyze it. The survival rates of several patient groups were compared using Kaplan-Meir curves and log-rank testing. To find predictors, the Cox's proportional hazards model is utilized.
Results: The incidence rate of the first ART regimen switching was 1.14 (95% CI: 0.88-1.17) per 100 person-years, with a median survival of 104 months. Viral load 150–1000 copies/mL and >1000 copies/mL (adjusted hazard ratio (AHR) = 4.3, 95% CI = 1.4–12.6 and 7.3, 95% CI = 2.6–20.3), compliance rate <85% (AHR = 5.9, 95% CI = 3-11.5), baseline CD4 count < 100 cells/mm3 (AHR = 2, 95% CI = 1.53-4), disclosure status (AHR = 1.8, 95% CI = 1.1-3.1) were significant predictors of initial regimen switch.
Conclusions: The incidence of initial regimen switching was considered to be low. Viral load from 150 to 1000 and >1000 copies/mL, adherence level <85%, baseline CD4 count <100 cells/mm3, and non-disclosure of HIV serostatus were independent of the initial ART regimen switch. was shown to be a predictor. All stakeholders should focus on patients with high viral loads, low CD4 counts, and poor adherence to reduce the number of HIV patients who fail treatment.
art: switching to second-line regimens: predictors: incidence rate: south ethiopia
Background:
The introduction of highly active antiretroviral therapy (HAART) is an important milestone in the history of HIV disease, dramatically reducing morbidity and mortality and improving the quality of life of people living with HIV/AIDS (PLWHA) (1). However, due to several factors, including regimen non-adherence, mutations with resistant strains of the virus have been found to date, and reports of MRD (multidrug-resistant) virus in treatment-experienced HIV patients are on the rise (2). As of the end of June 2020, 26 million people had access to antiretroviral therapy (3). According to 2016 global estimates, approximately 5.5% of patients worldwide received second-line treatment (4). Most people with HIV live in sub-Saharan Africa. Due to limited access to HIV diagnosis and treatment in these countries, AIDS-related morbidity and mortality remain among the highest in the world (5). By 2015, nearly 2 of her 100 HIV patients in sub-Saharan Africa were transitioning to secondary ART each year (6). In 2017, 1.5% of all ART patients in Ethiopia were second-line patients (7).The World Health Organization (WHO) recommends first- and second-line antiretroviral therapy for treatment-failed HIV patients to avoid drug resistance, severe immunosuppression, and increased morbidity and mortality. Recommends switching to therapy (ART) [8]. As ART utilization increases, the risk of treatment failure and resistance becomes more acute, and switching patients to second-line regimens is the preferred method for early detection of treatment failure, thereby reducing drug resistance. Reduce changes in cytotoxicity and improve clinical outcomes [9]. Second-line ART is a follow-up regimen used immediately after first-line therapy failure, where the goal of second-line therapy is to achieve complete viral suppression rather than complete viral suppression as in developed countries where multiple second-line therapies are used to prolong the survival of people living with HIV. Line options and salvage regimens are available, and early change is the norm (10). Secondary ART involves agents that maintain activity across the patient's viral strains, usually involving at least three active agents (11). According to the National AIDS Control Program, it consists of at least one non-nucleoside reverse transcriptase inhibitor, a protease inhibitor (12). Protease inhibitors are commonly used in combination with two nucleoside reverse transcriptase inhibitors to increase the therapeutic index and eliminate the possibility of ART resistance (13).The many reasons a patient switches her HAART therapy, plus interdependent and associated with switching from HAART therapy, include treatment failure, antiretroviral drug side effects, and poor adherence to therapy. There are also several factors (14). Treatment toxicity has been reported with all antiretroviral agents and is one of the most common reasons for switching, discontinuing, and non-adhering to medication (15). Most of the studies conducted in Ethiopia focused specifically on switching therapy rather than switching ART therapy and were mainly from the northern, western, and central parts of the country and from the southern part of the country to Ethiopia no studies have been conducted (16,17). Therefore, this study aims to assess the incidence and rates for switching from initial HAART therapy (first-line treatment) to second-line therapy and determine predictors from June 2010 to June 2020 at the Adola and Negele General Hospital, Guji District, Southern Ethiopia.
Methods and materials
Study setting and period:
The study was conducted at the Adult Outpatient ART Clinic of Adola and Negele General Hospital in the East Guji Zone, located 476 km and 596 km south of the county, capital Addis Ababa. Within the zone, there are four hospitals, two of which were established in 2019, namely Uraga and Bole primary hospitals. These hospitals have completed the study period (June 2010 to June 2020) and have not yet started pharmacy services in 2019 and are therefore not included in the study. The period is included in the survey. The treatment protocol was conducted according to the WHO ART treatment guidelines for HIV infection in adults and adolescents. Baseline assessments are performed at Week 0, followed by visits at Weeks 1, 2, 4, 8, 12,16, and 24. After 24 weeks of antiretroviral therapy, patients should return every 12 weeks (18). These hospitals now provide secondary care to the majority of the population of the East Guji zone, and patients are referred from almost all parts of the East Guji zone. The hospital is now offering first-, second, and third-line ART for him. The survey was conducted from May to June 2021.
Study design and study population
An institutional retrospective cohort study design was conducted. All eligible HIV/AIDS-infected adults who initiated HAART between June 2010 and June 30, 2020, at the Adola and Negele General Hospital outpatient ART clinic.
Inclusion and exclusion criteria
Inclusion criteria were adults (age 15 years or older) starting a HAART regimen between June 2010 and June 2020 at the Adola and Negele General Hospital outpatient ART clinic. However, the woman who received her ART solely for PMTCT did not have at least one follow-up appointment in the outpatient department, and the patient was transferred outside Adola and Negele General Hospital, with records sent from the study facility, and had unclear and incomplete records.
Sample size and sampling method
The Sample size was determined using the twice her population ratio formula for each target by Open EPI, version 7, open source. We use the following assumptions: 80% power [19]. The calculated sample size was 854. A simple random sample was used to select a given sample size. Inpatient card numbers/registration numbers were obtained from an electronic database. A patient record was then created using the card number. Patients who started ART but did not attend at least one of their follow-up visits were excluded from the study because the card was not transferred to the institution, excluding patients with incomplete baseline information. Next, assign all his MRNs his ID number and, using computer-generated random numbers, recruit his 854 data sets from study participants within 10 years from the follow-up period. The sampling frames were created for the rest of the dataset (Fig. 1).
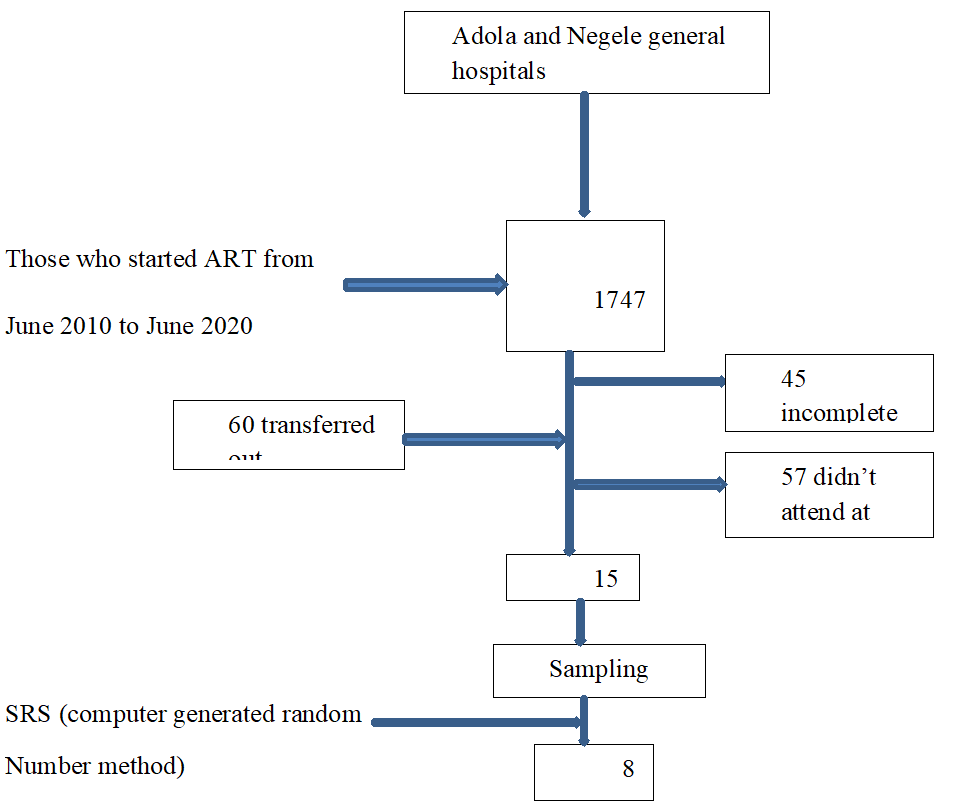
Figure 1: Schematic presentation of the sampling procedure for the study on incidence and predictors of initial antiretroviral therapy regimen switch among adult patients on antiretroviral therapy at Adola and Negele General Hospitals South Ethiopia, 2021
Data collection procedure :
Sociodemographic characteristics, baseline and follow-up clinical data, and laboratory data were collected from patient medical records using a structured and pre-tested data abstraction format. Candidate variables were identified from a patient registry developed by the Ethiopian Federal Ministry of Health (FMOH) [18]. A unique ART number was used to identify individual participants. The data abstraction form was adopted from the ART intake and follow-up forms from his ART clinic. It was pre-tested on 5% (43) of the total sample size found outside the Shakiso Health Center, a research facility. Patient admission forms, aftercare cards, ART registers, and electronic information databases served as data sources. Other clinical charts, including laboratory test results, were also used to document retroviral load and CD4 cell counts. Patients were followed retrospectively from the date of enrollment until the start of her HAART study. Investigators conducted training for data collectors and supervisors to familiarize themselves with data collection tools. The questionnaire was checked for completeness and the final checked questionnaire was returned to the principal investigator.
Operational definition:
% Adherence = (# of doses should have been taken - # missed doses)* 100
# of doses should have been taken
Adherence is considered optimal when its rate is greater than or equal to 95%.
Data quality control
Before starting the actual data collection, pre-testing was performed on randomly selected patients to check the clarity and completeness of the overall data collection format and methods. Possible modifications and changes to the data collection format were made based on available data and a review of previous literature. The data collector was his two BSc nurses at his ART clinic at the hospital, one supervisor was also from the clinic, and he is the pharmacy manager at his ART clinic. A one-day training session was conducted to familiarize both data collectors and supervisors with the data collection tools. Data were collected over 30 days. Data quality was maintained through intensive training of data collectors and supervisors on study objectives and data retrieval and extraction from patient records. Completed data collection tools were routinely checked for completeness of the information.
Data processing and analysis procedures :
Each questionnaire was reviewed for accuracy and consistency by the primary investigator and supervisor after data collection. Epi-Data version 4.6 was used to import the cleaned, modified, and coded data. From there, the data were exported to STATA version 15. To ensure that the data were distributed as expected, an exploratory data analysis was carried out. We employed descriptive statistics, such as mean, standard deviation, median, and interquartile range, after confirming the distribution of the data. Cohort characteristics were described in terms of frequencies and proportions. By dividing the number of individuals who altered their initial regimen throughout the follow-up period by the proportion of the subject's time at risk for the full follow-up period, the incidence of initial regimen change was estimated. In order to assess cumulative survival, life tables were employed. The log-rank test and Kaplan-Meier survival curves were used to compare the overall survival experiences of two or more groups and estimate mean times. After the Schoenfeld residual test and graphs validated the assumptions, the Cox's proportional hazards model was modified. To find correlations between each independent variable and the dependent variable, bivariate analysis was used. In the multivariate analysis, variables having P<0.25 values in the bivariate analysis were included. In addition, when choosing candidate variables for multivariate analysis, the history and findings of earlier investigations were taken into account. 95% CIs and P-values were used to determine the degree of connection and statistical significance after an inverse variable selection technique was performed to provide a list of the top predictors and adjusted hazard ratios. Each statistical test was considered significant at a P-value < 0.05.
Ethical considerations
Ethical clearance was obtained from Jimma University, the Institute of Health, Institutional Review Board (IRB/2021). Adola and Negele General Hospital administrators were informe of the study objectives by a letter of support from the Epidemiology Department and obtained approval before data collection. Patients' informed consent does not apply, as routinely available patient record data were used for the study. Confidentiality of information obtained from each study participant was ensured by omitting names and personal identifying information. Additionally, the collected data was kept secure throughout the research work process to limit access to the data by third parties.
Results:
Sociodemographic characteristics
In total, records from 854 HIV patients were selected and analyzed. The median patient age was 32.5 years (IQR = 27–40), with the majority 345 (40.4%) in her 25–34 year group. More than half of the patients, 448 (52.5%), were women, and approximately 433 (50.7%) were Orthodox Christians. Regarding the patient's educational background, about 489 (57.3) had primary education or higher. A total of 541 (63.3%) were urban residents (Table 1).
Table 1: Baseline sociodemographic Characteristics of HIV positive Adult patients at the initiation of ART at Adola and Negele General Hospital from June 2010 to June 2020
|
Characteristics |
Categories |
Total (No. (%)) |
|
Age |
15-24 |
131(15.3) |
|
25-34 |
345(40.4) |
|
|
35-44 |
269(31.5) |
|
|
>45 |
109(12.8) |
|
|
Sex |
Male |
406(47.4) |
|
Female |
448(52.6) |
|
|
Marital status |
in a couple |
493(57.3) |
|
single |
361(43.7) |
|
|
Religion |
Orthodox |
433(50.7) |
|
Muslim |
68(8) |
|
|
Protestant |
337(39.5) |
|
|
Catholic |
16(1.9) |
|
|
Educational status |
No formal education |
365(42.7) |
|
Primary education and above |
489(57.3) |
|
|
Occupation |
Farmer |
216(25.3) |
|
Merchant |
215(25.2) |
|
|
Government employee |
62(7.3) |
|
|
Daily laborer |
105(12.3) |
|
|
Student |
54(6.3) |
|
|
Housewife |
202(23.6) |
|
|
Residence |
Urban |
541(63.3) |
|
Rural |
313(36.7) |
|
|
Disclosure status |
Yes |
469(54.9) |
|
No |
385(45.1) |
Clinical, immunological, virological, and therapeutic-related features
The first major HAART regimen they prescribed was the combination of tenofovir (TDF) + lamivudine (3TC) + efavirenz (EFV), 537 (62.9%), followed by zidovudine (AZT) + lamivudine (3TC) + nevirapine (NVP) and Zidovudine (AZT) + Lamivudine (3TC) + Efavirenz (EFV). The majority (753 (88.2%) of patients received CPT prophylaxis (Table 4) 398 (46.6%) were in WHO clinical stage II at the initiation of ART was 56.00 kg quartile Range (IQR): 50-61. Of a total of 854 patients, 108 (12.6%) met treatment failure criteria, and only 52 (48%) patients transitioned to second-line ART therapy. The reason for not switching is not documented. The median baseline CD4 cell count at the initiation of ART was 409.50 cells/mm3 (IQR: 236.75-600.00). The majority of her study participants, 619 (72.5%), had undetectable viral loads and more than 80% (686) recorded good adherence (Tables 2 and 3).
Table 2: Virological, Clinical, and Immunological characteristics of HIV-positive Adults on First line ART at Adola and Negele General Hospital from June 2010 to June 2020
|
Characteristics |
Categories |
Total (No. (%)) |
|
Viral load |
Undetectable |
619(72.5) |
|
150-1000 |
130(15.2) |
|
|
>1000 |
105(12.3) |
|
|
Baseline WHO stage |
Stage I |
236(27.6) |
|
Stage II |
398(46.6) |
|
|
Stage III |
175(20.5) |
|
|
Stage IV |
45(5.3) |
|
|
BMI |
<18.5 |
151(17.7) |
|
18.5-24.5 |
660(77.3) |
|
|
>24.5 |
43(5) |
|
|
Nutritional status |
Not malnourished |
707(82.8) |
|
Moderate |
120(14) |
|
|
Severe |
27(3.2) |
|
|
Functional status |
Working |
715(83.7) |
|
Ambulatory |
102(11.9) |
|
|
Bedridden |
37(4) |
|
|
CD4+ |
<100 |
81(9.5) |
|
100-350 |
286(33.5) |
|
|
>350 |
487(57) |
Table 3: Treatment-related characteristics of HIV-positive Adults on First line ART therapy at Adola and Negele General Hospital from June 2010 to June 2020
|
Characteristics |
Categories |
Total (No. (%)) |
|
Type of initial HAART regimen |
TDF-3TC-DGT |
71(8.3) |
|
TDF-3TC-EFV AZT-3TC-NVP AZT-3TC-EFV D4T-3TC-EFV |
537(62.9) 169(19.8) 58(6.8) 19(2.2) |
|
|
Adherence level |
>95% 85-94% <85% |
686(80.3) 89(10.4) 79(9.3) |
|
Treatment change |
Yes No |
293(34.3) 561(65.7) |
|
Treatment failure |
Yes No |
105(12.3) 749(87.7) |
|
CPT prophylaxis |
Yes No |
753(88.2) 101(11.8) |
|
History of TB |
Yes No |
127(14.9) 727(85.1) |
|
ARVs adverse effect |
Yes No |
152(17.8) 702(82.2) |
Change in the incidence of first-line ART regimens to second-line
Median follow-up for IQR (21-59) was 44 months, with a total of 35,444 person-months (2919.36 person-years) of observation. The overall incidence of first ART regimen switching was 1.88 (95% CI: 0.88, 2.89) per 100 years of follow-up.
Overall, 55 (6.4%) persons switched to second-line therapy, the most common second-line regimen to which people switched was tenofovir, lamivudine, and ritonavir-boosted atanavir 28(50.9%) followed by stavudine, lamivudine, and ritonavir-boosted atanavir 18(37.7%). Among the reasons for regimen switch, virologic failure was the commonest reason for the initial regimen switch, which accounts for 61.8% of cases and contributes 15.9 (95% CI: 11.33–22.2) per 100 PYs (Figure 2).
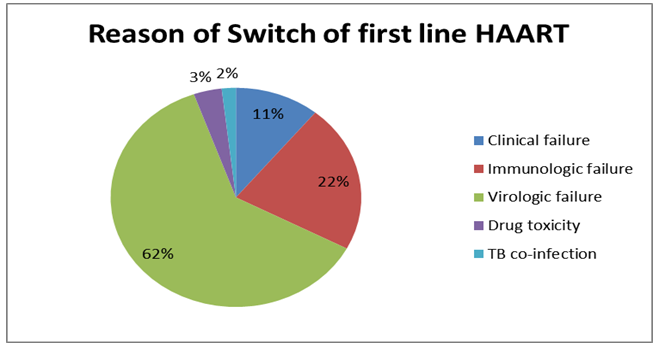
Figure 2: Reasons of switch among those patients who switched to second line ART in Adola and Negele General Hospital from June 2010 to June 2020
The cumulative probability of surviving on an initial regimen to the end of 2 years was 96%; to the end of 4 years was 83%; to the end of 6 years was 75% and to the end of 8 years was 37% (Table 4).
Table 4: Life table on the incidence of Initial ART Regimen switch and predictors Among Adult HIV patients on the First line HAART regimen in Adola and Negele General Hospital from June 2010 to June 2020
|
Interval Start Time |
Number Entering Interval |
Number Withdrawing during Interval |
Number Exposed to Risk |
Number of Terminal Events |
Proportion Surviving |
Cumulative Proportion Surviving at End of Interval |
|
0 |
854 |
222 |
743.000 |
9 |
.99 |
.99 |
|
2 |
623 |
234 |
506.000 |
14 |
.97 |
.96 |
|
4 |
375 |
313 |
218.500 |
29 |
.87 |
.83 |
|
6 |
33 |
28 |
19.000 |
2 |
.89 |
.75 |
|
8 |
3 |
2 |
2.000 |
1 |
.50 |
.37 |
The overall Kaplan–Meir survival function estimate showed that most of the initial ART regimen switch occurred in later months of follow-up, with a median survival of 104 months (Figure 3).
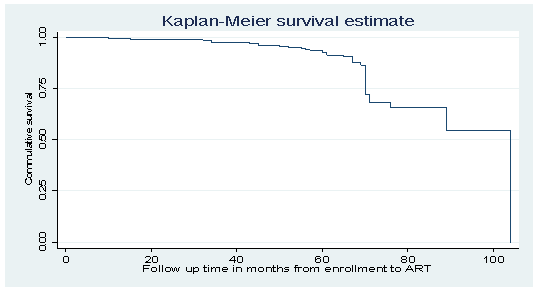
Figure 3. Kaplan-Meier curve of surviving Initial First line ART regimen for HIV-positive Adults at Adola and Negele General Hospital, from June 2010 to June 2020
Time to switch to second-line antiretroviral treatment:
Fifty-three (50.5%) out of 105 patients who fulfilled the criteria of treatment failure switched to the second line with delays of greater than six months with IQR (6-11 months) from the date of the confirmed treatment failure to the date of switch to second-line ART (Table 5).
Table 5: time to switch to second ART regimen from date of treatment failure to date of regimen switch among treatment-failed patients at Adola and Negele General Hospital from June 2010 to June 2020
|
Number of total treatment failures = 105 |
||
|
Time to switch |
Frequency |
Percent |
|
Less than 6 months |
0 |
0 |
|
Greater than 6 months |
53 |
50.5 |
Predictors of Initial first-line ART Regimen switch to the second line
Bi-variable Cox regression analysis showed that marital status, Residence, Baseline WHO stage Viral load, Adherence level, disclose to close family member, Treatment change, Baseline BMI, Baseline CD4+, Nutritional status, ARVs adverse effects, Functional status, CPT prophylaxis, and History of TB are candidate variables for multivariable Cox regression. However, in the Multivariable Cox regression analysis, viral load, Baseline CD4+, Adherence level, and disclosure status remained statistically significant predictors of the incidence rate of the initial regimen switch (Table 6).
Table 6: Bi-variable and Multi-variable Cox Regression Analysis of Incidence rate of Initial ART regimen switch Among Adults on first Line HAART at Adola and Negelle General Hospitals, Southern Ethiopia from June 2010 to June 2020
|
Patient cohort |
Number of switches |
PYFU |
Rate/100PY |
bivariate Analysis |
Multivariate Analysis |
|||
|
Hazard ratio(95% CI) |
P-Values |
Adjusted HR(95% CI) |
P-values |
|||||
|
Viral load |
Undetectable |
0 |
2142.73 |
0 |
Ref. |
|
Ref. |
|
|
150-1000 |
5 |
423 |
1.2 |
3.4(1.8-6.34) |
<0.001 |
3.3(1.8-6.3) |
0.009 |
|
|
>1000 |
50 |
387.91 |
12.9 |
9.4(5.6-15.7) |
<0.001 |
7.3(2.6-20.3) |
0.001* |
|
|
Adherence level |
>95% |
1 |
2342.3 |
0.043 |
Ref. |
|
Ref. |
|
|
85%-95% |
12 |
308.83 |
3.9 |
3(1.6-5.8) |
0.06 |
2.2(1.1-4.5) |
0.058 |
|
|
<85% |
42 |
302.51 |
12.9 |
6.5(3.3-12.7) |
0.001 |
5.9(3-11.5) |
0.001* |
|
|
Baseline CD4+ |
<100 |
44 |
344.66 |
12.8 |
7.7(4-14.6) |
0.001 |
2(1.53-4) |
0.039* |
|
100-350 |
9 |
970.89 |
0.93 |
1.4(0.65-2.98) |
0.39 |
7(4-14) |
0.57 |
|
|
>350 |
2 |
1638.09 |
0.12 |
Ref. |
|
Ref. |
|
|
|
D Disclosure status |
Yes |
12 |
1644.84 |
0.73 |
Ref. |
|
Ref. |
|
|
No |
43 |
1308.8 |
3.3 |
1.7(1-2.9) |
0.048 |
1.8(1.1-3.1) |
0.025* |
|
Comparison of Survival Probability among Categories of Covariates
Patients with high viral load are at higher risk of regimen switch compared to those with low viral load. As shown the figure below, having an adherence level below 85% is the risk of switching patients from the initial first-line ART regimen to the second-line ART regimen. Patients with Baseline CD4 count below 100 cells/mm3 are at higher risk of switching their initial first-line ART regimen to a second-line ART regimen compared to those with a baseline CD4 count above 350 cells/mm3. Disclosure of HIV serostatus reduces the risk of switching from an initial first-line ART regimen to a second-line ART regimen compared with patients who do not disclose HIV status.
Discussion :
In low-income countries, the choice of appropriate regimens is limited, so a well-performed first-line of his ART is very important. Evaluation of the continuous switching rate of the first regimen and its predictors can help the patient to maintain her first ART regimen for as long as possible [27]. In this study, the incidence of first ART regimen switching was 1.14 per 100 years of follow-up (95% CI: 0.88, 1.7), and median follow-up at 44 months his IQR (21-59) was. If all patients on first-line therapy had access to viral load testing, the conversion rate to second-line ART could be higher. The main factors that influenced the incidence of the first ART regimen switch were treatment change, viral load, her CD4+ at baseline, adherence level, and disclosure status. The rate of switching to second-line ART in this study which is 1.8 per 100 PYFU is in line with the results of some other studies, especially from programs that have no routine viral load monitoring[(6,19,28,29)]. This is lower than a study conducted in Resource limited setting supported by Medecins Sans Frontiers (MSF) 48/100PYs[30], in Uganda, it was 49/100PYs[31], in Mali, it was 3.3/100PYs[27]. This lower rate might be due to the difference in the median follow-up period which was 20 months in Medecins Sans Frontiers (MSF) supported countries, 16.8 months in Uganda, and 15 months in Mali but 44 months in this study, and this may be associated with as person’s time of observation increase there be decreases incidence rate. Another reason may be physicians are reluctant to switch ART when treatment options are limited and also may be that our study assessed ten-year data on the time at which second treatment was not decentralized to General Hospitals. This finding is also lower than the study done in another resource-limited setting with access to viral load monitoring (2.4/100PYs). A possible reason may be the accessibility of viral load to help identify more patients with treatment failure [32]. In this study, regular viral load monitoring, which allows early detection of virologic failure and indicates the need for regimen switching, is not routine. In this study, toxicity was one of the reasons for switching from first-line HAART therapy to second-line therapy. This can be observed in several other studies [(32,27)]. Tuberculosis co-infection accounts for 1.8% of patients switched to secondary ART. Treatment failure can lead to tuberculosis. The risk of virologic unsuppression may also be increased by his concomitant ART and tuberculosis treatment due to impaired adherence and pharmacokinetic drug interactions [33]. Patients receiving ART for active tuberculosis should be prioritized for viral load monitoring and compliance support. It is important to strengthen interventions to prevent tuberculosis during ART. B. Isoniazid prophylaxis and infection control in healthcare settings. A possible explanation for this could be an increase in viral copies. This can adversely affect treatment response by compromising immunity and contributing to the side effects of the double burden of tuberculosis and HIV, which approved his CDC guidance on HIV treatment and strategies. [12].In this study, in all patients transitioning to a second-line ART regimen, there was a delay of 6 months or more in transitioning to a second-line ART regimen after documented treatment failure, consistent with reports from other institutions
(31). Delays in transitioning patients to secondary ART; highlight the lack of robust systems needed to manage chronic diseases such as HIV in public health settings. The results of this study showed that viral loads in patients with viral loads between 150 and 1000 copies/mL and >1000 were significant predictors of the incidence of first regimen switching, with a 2.2-fold and 7.3-fold increased risk of 1, respectively. The 2nd administration, exhibits the line ART scheme. A detectable high viral load is the result of poor disease progression. As the viral load increases, the disease is considered progressive, and eventually, the patient becomes more susceptible to treatment failure and declining CD4 levels in her, later leading to the development of opportunistic infections. This finding is supported by studies conducted in Dakar, Senegal, and Rakai, Uganda [(31,29)]. Possible reasons could be patients with high viral load, advanced-stage disease, low CD4 counts, OIs, and possibly other chronic diseases. This increases the likelihood of taking additional medications and may also lead to drug interactions, the occurrence of side effects, and poor treatment response. More extensive viral load monitoring in resource-limited areas Good access should be considered a priority for guiding physicians in ART management and optimizing the use of limited treatment options. Patients with low baseline CD4+ counts (<100 cells/mm3) on ART were more likely to switch initial therapy at any time compared to patients with high baseline CD4+ counts (>350 cells/mm3) were twice as high. It is well known that CD4 counts are inversely related to viral replication and viral load. When the patient's immune status is compromised, the rate of viral replication increases compared to immunocompetent counterparts. In addition, immune-compromised patients are susceptible to various opportunistic infections that maintain a vicious cycle of immunity and viral replication, and failure of immune reconstitution should also be a surrogate marker of virologic failure (34). This finding is supported by studies conducted in Myanmar, Dakar, Senegal, Rakai, Uganda, and sub-Saharan Africa (31,19,29,35). A possible explanation is that patients who initiate ART treatment at advanced stages are more likely to develop the worse disease, develop side effects, and are at higher risk of treatment failure. This is leading to changes in treatment regimens, and drug interactions likely to be more susceptible to other forms of opportunistic infection. Virologic and immunological responses are slower than those with higher CD4 counts. Patients with <85% adherence are 5.9 times more likely to switch to a second regimen of ART than those with >95% adherence. This finding is consistent with studies conducted in southwestern Uganda and Myanmar (19, 36). There is general agreement that adherence issues are the most important concern for ART users and therefore poor adherence increases the risk of virologic failure.Evidence suggested that patients are more likely to acquire drug resistance and low immunity when adherence levels are below 95%, and that the CD4 count considerably drops in these individuals. High adherence rates are typically required to achieve therapeutic success, and it is possible for treatments to cause immunological failure [37]. This results in virological failure and provides the right environment for viral replication. The rationale for the regimen changes could later be due to the fact that patients who skip a clinic visit, are not fully retained in care, or have poor drug adherence are more likely to have treatment failure. A patient's loss from care is a proxy for poor adherence and interruption of therapy, according to published evidence that patients who miss a clinic visit, are not fully retained in care, or have poor drug adherence are more likely to experience treatment failure and loss to follow-up at least once[38, 39]. Social professionals or counselors need to analyze this group of patients further in order to understand why they are not receiving care, and the proper help needs to be given. The results of this study indicate that disclosure status emerged as a significant predictor of the incidence of first regimen switching, with patients who did not initially disclose their status more likely to switch regimens than those who did rate was 1.8 times higher. This is because patients who disclose their condition have the opportunity to access social networks for advice, emotional support, information, and other social resources, making them more aware of the appropriate use of ART medications. These benefits are lost in patients who do not disclose this.
Limitations of research
The retrospective nature of this study may be a limitation as the accuracy of the analysis depends on the completeness of the records, thus information bias may have occurred due to underreporting/missing data elements. Other limitations are assessing adherence based on records without using standardized questionnaires and excluding transfer patients. These analyses did not include patients who lost follow-up, which may have contributed to treatment failure and ultimately lead to treatment switching.
Conclusion :
In the current study the incidence of early regime change is low. Viral load <150–1000 copies/mL to <1000 copies/mL, adherence level <85%, baseline CD4 count <100 cells/mm3, and non-disclosure of HIV serostatus was shown to be an independent predictor of initial ART regimen switch.
Recommendation :
Patients should inform family and relatives of their HIV status and adhere to treatment. Providers should encourage patients initiating ART to disclose their HIV status. Also, special caution should be exercised in patients with baseline CD4 count <100 cells/mm3, adherence <85%, viral load 150-1000 copies/ml, and viral load >1000 copies/ml/ml. It is recommended that you pay. Providers should timely switch patients who fail initial HAART therapy. Those in a position to develop guidelines should consider decentralizing viral surveillance of HIV patients. Researchers should work with stakeholders. Prospective follow-up including socioeconomic factors, loss of follow-up, outsourced patients, and stigma-related factors to better understand the predictors of her switching from first-line to second-line ART therapy Conducting research is recommended.
Abbreviations and Acronyms
|
3TC |
Lamivudine |
|
ALT |
Alanine aminotransferase |
|
AST |
Aspartate aminotransferase |
|
ATV/r |
Atazanavir/ritonavir |
|
AZT |
Stavudine |
|
cART |
Combined Antiretroviral Therapy |
|
CD4 |
Cluster Differentiation T-lymphocyte |
|
HAART |
Highly Active Antiretroviral Therapy |
|
HIV |
Human Immune Virus |
|
HIVDR |
Human Immune Virus Drug Resistant |
|
MRD |
Multiple Drug Resistant |
|
NVP |
Nevaprine |
|
PI |
Protease Inhibitors |
|
PLWHA |
People living with HIV/AIDS |
|
PMTCT |
Prevention Mother To Child Transmission |
|
PY |
Person-Years |
|
SPSS |
Statistical package for Social Science |
|
TB |
Tuberculosis |
|
TDF |
Tenofovir Disoproxil Fumarate |
|
WHO |
World Health Organization |
Consent for publication:“Not applicable”
Availability of data and materials
Data essential for the conclusion are included in this manuscript. Additional data can be obtained from the corresponding author on a reasonable request time.
Funding :No funder for specific study.
Competing interest: We declare that we do not have conflicts of interest on all activities pertaining this research work.