
Ali Sreij1*, Anthony Nasr2 and Raja Sawaya1
1Department of Neurology, Clinical Neurophysiology Laboratory, American University of Beirut Medical Center, Beirut, Lebanon.
2Department of Diagnostic Radiology, American University of Beirut Medical Center, Beirut, Lebanon.
*Corresponding Author: Ali Sreij, Department of Neurology, Clinical Neurophysiology Laboratory, American University of Beirut Medical Center, Beirut, Lebanon.
Received Date: March 25, 2024
Accepted Date: May 08, 2024
Published Date: May 24, 2024
Citation: Ali Sreij, Anthony Nasr and Raja Sawaya. (2024) “A Misinterpreted Case of Froin’s Syndrome.”, International Journal of Medical Case Reports and Medical Research, 2(4); DOI: 10.61148/2994-6905/IJMCRMR/036.
Copyright: © 2024. Ali Sreij. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Froin’s syndrome occurs when CSF protein levels are extremely high. We describe a case that was misdiagnosed as Froin’s syndrome as the CSF protein was 15g/L. The relevant investigation did not reveal any pathology or explanation for the elevated CSF protein. Repeat CSF analysis within one month was normal. We believe that the needle in the first spinal puncture was misguided into a renal cyst in proximity to the spinal canal.
froin’s syndrome; elevated CSF protein; lumbar puncture; CSF stagnation; renal cyst
Introduction:
Froin’s syndrome, first described in 1903, occurs when the CSF is found to be xanthochromic with extremely high CSF protein level (> 5g/L) causing it to become hypercoagulable. Froin’s syndrome results from the blockage of the spinal canal causing an isolated CSF space caudally. Venous congestion or inflammation in this region results in blood plasma products leaking into the subarachnoid space raising the CSF protein. [1,2] Froin’s syndrome is usually caused by a tumor, infection, or inflammation obstructing normal CSF flow. [1,2] Pseudo-Froin’s syndrome has also been described in cases with the same CSF findings, but no evidence for a blockage of the spinal cord. Pseudo-Froin’s syndrome has been described in patients with paraplegia, and stagnation of the CSF below the level of the spinal cord injury. [2,3] It has also been described in patients with meningitis, and destruction of the arachnoid vilae causing stagnation of the CSF in the spinal canal. This phenomenon causes a passive or active diffusion process, resulting in hyperprotenosis, and hypercoagulation of the CSF. [2,4]
Froin’s syndrome has been described in patients with spinal cord abscesses, tumors, multiple myeloma, leptomeningeal carcinomatosis, epidural hematoma with cord compression, and tuberculous meningitis with tuberculomas of the spinal cord. The levels of CSF protein reported range between 12 g/L up to 38 g/L. [3-7]
Case Report:
We describe a case of a 77-year-old, previously healthy, man who presented because of progressive dementia, urinary incontinence, and unsteady gait. MRI of the brain revealed moderately enlarged ventricles associated with diffuse cortical atrophy predominantly in the temporoparietal regions bilaterally. The clinical and radiological pictures were suggestive of normal pressure hydrocephalus. The patient underwent a CSF tap of 50 ml as a diagnostic test. The tap was non-traumatic but the CSF was xanthrochromic (Figure. 1A). The CSF protein level was 15.56 g/L with the absence of white blood cells, a normal sugar level, and absence of malignant cells by cytology. The diagnosis of Froin’s syndrome was established.
A search for the cause of the elevated CSF protein was performed. The patient underwent an MRI of the cervical, dorsal, and lumbosacral cord in search of a spinal block. The MRI images were normal. Screening hematology and chemistry blood studies were also normal. PET-CT body revealed only some uptake in the proximal colon. Colonoscopy was negative. FNA of a thyroid nodule did not reveal any malignancy. CT's body did not show any infectious or malignant disease in the chest, abdomen, or pelvis. A repeat spinal tap was performed around one month later for confirmation of the previous findings. The CSF was clear, and the protein level was normal at 0.41 g/L. (Figure. 1B)
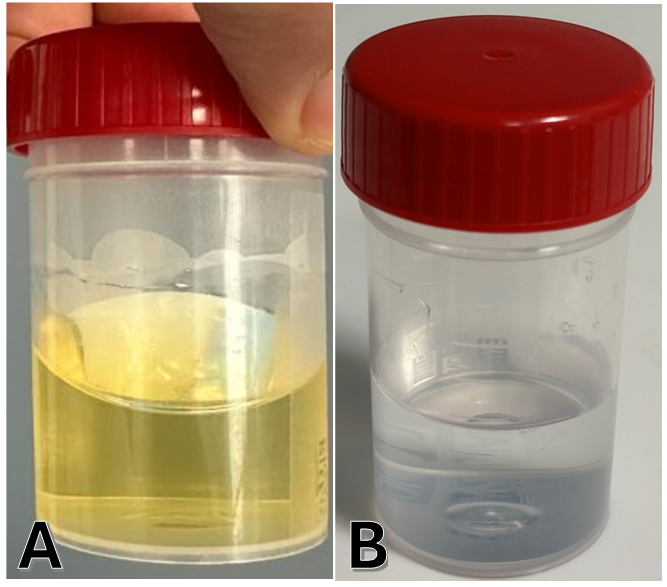
Figure 1: Cerebrospinal fluid collected (A) January 1, 2024 (B) February 28, 2024.
Discussion:
Froin’s syndrome by definition is caused by a blockage of the spinal cord causing stagnation of the CSF caudal to the lesion. [1] MRI of the brain and spine, and whole body PET-scan in our patient failed to reveal any obstruction of the CSF in the neural axis. Pseudo-Froin’s syndrome is the result of stagnation of the CSF in the spinal cord secondary to abnormal circulation. This can result from stenosis at a cranial level of the cord [4], destruction of the arachnoid villa by infection [7], or stagnation of the CSF in paralyzed patients. [2]
Our patient had no stenosis at any level of the cord, no meningitis clinically with zero CSF white blood cell count, and no evidence of stagnation of the CSF circulation.
The abnormality in the CSF findings in this patient is first the severely elevated CSF protein level, in the first tap, with no explanation, and second the normalization of the CSF protein spontaneously one month later.
A review of the lumbar puncture technique performed in parallel with a review of the abdominopelvic CT scan and MRI of the lumbosacral spine raised the suspicion that the first spinal tap could have been mistakenly directed 30˚ away from the midline to the left side, given the patient’s lumbar scoliosis, possibly sampling fluid from the large peri-renal cyst on that side. (Figure.2)
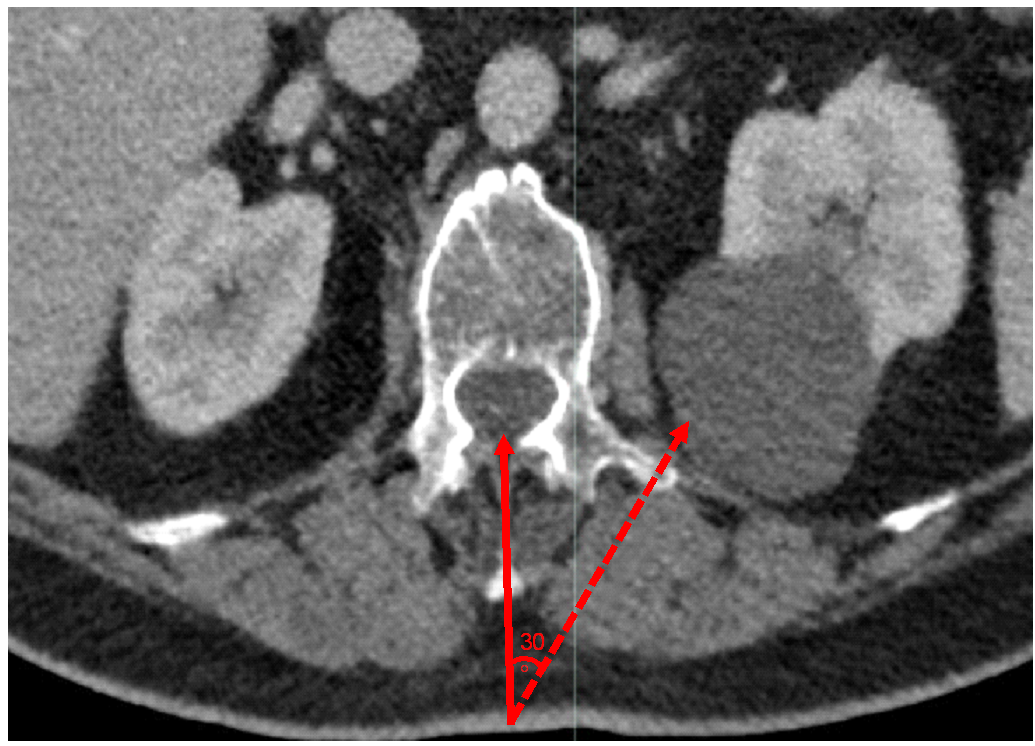
Figure 2: Axial contrast enhanced CT of the abdomen and pelvis at L1-L2 level showing a left simple renal cyst adjacent to the vertebra. The figure demonstrates that if the point of entry was 30 degrees angulated from the midline, fluid would be drawn from the renal cyst instead of the spinal canal.
This interpretation is because the protein level in the patient’s urine was 1.26 g/L, meaning the tap was not in the renal calyx or renal parenchyma. On the other hand, the protein level in renal cysts has been described to be between 7 to 28 g/L placing the first CSF protein level in our patient in this domain. [8]
We conclude that the first lumbar puncture was probably 30˚ to the left of the midline sampling fluid from the left peri-renal cyst measuring 92 x 60 x 85 mm. The direction was forced because of the patient’s moderate lumbar scoliosis (convex to the right). The second lumbar puncture revealed the correct CSF protein level in this patient.
Conclusion:
We recommend that physicians should always investigate etiologies for very high CSF protein levels and search for a pathology blocking CSF flow and causing its stagnation. It is only when no such pathology is found that physicians should search for very unusual causes of elevated protein in their sample. An example of such an exception is our case where the sample was from an adjacent large renal cyst.