Ariel Benor*, Emily Tetelman
Maimonides Medical Center, Department of Obstetrics & Gynecology
*Corresponding author: Ariel Benor, Maimonides Medical Center, Department of Obstetrics & Gynecology.
Received: April 16, 2021
Accepted: April 20, 2021
Published: April 23, 2021
Citation: Ariel Benor, Emily Tetelman (2021) “A Case of Uterine Torsion Mimicking Pulmonary Embolism”, International J of Clinical Gynaecology and Obstetrics, 1(1); DOI:http;//doi.org/04.2021/1.1019.
Copyright: © 2021 Ariel Benor. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
BACKGROUND: Uterine torsion is not usually considered in the ante/intrapartum setting due to its rarity and clinical ambiguity, but it can lead to serious consequences. Despite its documented occurrence in the literature, scant information can be found regarding diagnosis and management.
CASE: A 41 year-old, multiparous woman in the early-preterm period presented to the hospital with preterm premature rupture of membranes (PPROM) and a breech fetus with no known uterine anomalies and an otherwise uncomplicated antenatal course. Her hospital course was complicated by desaturation, chest pain and diaphoresis, raising suspicion of pulmonary embolism (PE), which was eventually ruled out. However, after observing a non-reassuring fetal status, the patient was taken to the operating room for a cesarean section due to malpresentation. The diagnosis of torsion was eventually made intra-operatively when her uterus was found to be rotated 180 degrees on its longitudinal axis (Figure 1).
CONCLUSION: Uterine torsion is a rare event that can lead to serious maternal/fetal morbidity and mortality, if not discovered and treated early. It is difficult to diagnose clinically, as there are no specific signs or symptoms, but it can be diagnosed with MRI and ultrasound. It can occur during pregnancy as well as other times. If it occurs during pregnancy, the fetal outcome is dependent on the degree of torsion and gestational age.
Teaching Points:
CASE: A 41 year-old G5P3104 @33w2d presented to the labor & delivery triage complaining that her “water broke.” Her obstetric history was complicated by one preterm delivery at 35 weeks due to preeclampsia. In that pregnancy, she was induced and had an uncomplicated vaginal delivery. Her three subsequent pregnancies were full-term normal spontaneous vaginal deliveries. Her medical history was significant for morbid obesity (BMI 40) and hypothyroidism, for which she was taking levothyroxine 150 mcg daily and doubling her dose to 300 mcg twice weekly to account for the increased pregnancy needs. She was also taking 81 mg aspirin orally daily to prevent preeclampsia due to her history of preeclampsia fifteen years prior. Her antenatal course was uncomplicated: the fetal anatomy scan was normal, except for an incidental finding of elevated right uterine artery waveform indices (S/D ratio 3.0).
On admission, her vital signs were stable: blood pressure 120/69, heart rate 56, respiratory rate 16, and she was afebrile. Her cervix was closed, 60% effaced, firm and posterior. Her membranes had ruptured approximately one hour prior to presentation. Her abdomen was soft, non-tender, and fetal movements were palpable. The nitrazine test was positive, pooling of clear fluid was noted in the vagina on speculum examination, and a trans-abdominal sonogram revealed an amniotic fluid index of 3.5 cm with no 2x2 cm pocket. The fetus was in a breech presentation, and the biophysical profile was 6/8, due to oligohydramnios. The fetal heart rate tracing was category I, and no contractions were noted. She was admitted to the antepartum service for management of PPROM. She was started on antibiotics and betamethasone.
On the second hospital day, she had a sonogram, which revealed that the fetus had an estimated fetal weight (EFW) in the 85%ile and an abdominal circumference (AC) in the 94%ile. The amniotic fluid index (AFI) was 1.9 cm, and the umbilical artery doppler velocimetry indices were within normal limits.
A turn of events happened on the following day. The sonogram revealed anhydramnios with a biophysical profile score of 6/8. At that point, the patient was tachycardic with a pulse in the 120s and diaphoretic, de-saturating to 89% on room air, and complaining of “feeling hot.” Her thyroid-stimulating hormone level was within normal limits, and she had been placed on DVT (deep-vein thrombosis) prophylaxis on admission with 10,000 units of subcutaneous heparin, twice daily. An EKG (electrocardiogram) revealed sinus tachycardia, and cardiac enzymes were normal. Her oxygen saturation improved with two liters oxygen via nasal cannula. A lower extremity Doppler was performed to rule out DVT. Although it was negative, a CT angiography of her chest was performed to rule out a pulmonary thromboembolism. This, too, was normal. On the next day, however, the sonogram report again revealed anhydramnios. However, this time the fetus received a biophysical score of 2/8, because only breathing was seen, but no tone or movement visualized. The mother’s abdomen was still soft and non-tender, and she did not have any vaginal bleeding. The decision was made to proceed with a cesarean section due to non-reassuring fetal status in the setting of preterm premature rupture of membranes in the early preterm period.
Upon entry into the peritoneal cavity, the posterior wall of the uterus was visualized, and the uterus was found to be dextro-rotated 180 degrees. The surgeons were unable to de-torse the uterus, so a transverse uterine incision was made in the lower segment of the posterior wall. After delivery of the baby and repair of the hysterotomy, the uterus was de-torsed and replaced to its correct anatomical position, as can be seen in Figure 1. No other intra-abdominal anomalies were noted. The placenta was removed without difficulty, and there were no signs of abruption. It was not sent to pathology.
Fortunately, the baby was born with reassuring findings. His one and five-minute APGARs were 7 and 8. The arterial pH was 7.21 with a pCO2 of 75, bicarbonate level of 21 and base deficit of 1.6, which were not significantly abnormal. The neonate was transferred to the NICU (neonatal intensive care unit) due to its prematurity and requirement for CPAP (continuous positive airway pressure). He was discharged two weeks later after meeting the appropriate milestones for a premature neonate.
The mother recovered well after the operation and reported improvement in her clinical status. Her symptoms of chest pain and diaphoresis had resolved soon after the operation, and her pulse and oxygen saturation levels had normalized post-operatively. There were no measures done to prevent the torsion from re-occurring, and the patient was counseled that it might happen again.
DISCUSSION AND LITERATURE REVIEW:
While there have been other case reports of uterine torsion in pregnancy (Duplantier et al., Jenson et al.), there are additional learning points that arise from this one. Torsion of the pregnant uterus is defined as rotation more than 45 degrees around the long axis of the uterus (Dua 2006). It is a rare and potentially life-threatening condition. The complications include placenta abruption, fetal demise, maternal shock and death. Pre-operative diagnosis is difficult, and the management must be individualized. It can occur in all trimesters of pregnancy as well as in the non-pregnant state (Jenson 1992, Kremer 1989).
The cause of uterine torsion has not been clearly elucidated; however, uterine asymmetry, from fibroids or Müllerian anomalies, has been identified in most cases (Farhadifar 2013). Other predisposing factors include fetal malpresentation, pelvic adhesions, ovarian tumors, and abdominal or ligamentous laxity. Only 16% of cases occur without discernible cause (Kovavisarach 1999). One study of MRIs following low transverse cesarean section suggested that, in rare instances, poor isthmic healing might result in suboptimal restoration of normal cervical length, resulting in an elongated cervix with structural weakness and angulation, leading to torsion (Kawakami et al., 1994). Uterine torsion resulting from abdominal trauma has also been reported (Duplantier et al., 2002). The clinical presentation of uterine torsion is non-specific. Most women in the literature with torsion presented with abdominal pain that can imitate the more common obstetric emergencies, such as scar dehiscence, uterine rupture or placental abruption (Kovavisarach). They also may have gastrointestinal distress, such as nausea, vomiting, diarrhea and abdominal distention. In pregnancy, obstructed labor, abdominal pain, uterine hypertonus, vaginal bleeding, and maternal shock are common signs and symptoms, with abdominal pain being most common. Other clinical signs include a twisted vaginal canal or urethral displacement. Eleven percent of cases are asymptomatic (Jenson, 1992). Variable symptomatology and lack of a specific diagnostic sign make the diagnosis extremely difficult without direct visualization during surgery. Torsion presenting in labor may manifest itself by failure to dilate or fetal distress, due to reduction in uterine blood flow (Dua, 2005). Nicholson et al. suggested the use of pelvic MRI to diagnose uterine torsion, which may show an X-shaped configuration of the upper vagina. Maternal prognosis depends on the stage of pregnancy and degree of rotation.
In terms of management, patients with acute symptoms should have a laparotomy. In early pregnancy, the uterus can be manually untwisted along with correction of any precipitating factors, like myomectomy or ovarian cystectomy. In cases with uterine necrosis or thrombosis of blood vessels, hysterectomy should be considered. Mortality rates are highest in the fifth to sixth months of pregnancy (17%) and decrease as gestation advances. Torsion of 180-360 degrees historically carries a 36% mortality rate, although only one death has been reported since 1960 (Guie, 2005). “In cases of torsion recognized at term, manual correction followed by delivery of the fetus by Cesarean section is the treatment of choice. In cases where correction is not possible, a deliberate posterior hysterotomy may be done.” (Dua) Patients with posterior wall incisions should have repeat sections in the future, as the risk of rupture is unknown. Bilateral plication of the round ligaments can be done to prevent immediate postpartum recurrence (Pelosi, 1998).
In this case, we found no obvious cause or risk factors for uterine torsion, e.g. the patient had no prior uterine scar, fibroids or other pelvic pathology. The clinical picture of desaturation, chest pain and diaphoresis led the provider to consider other etiologies, including pulmonary embolism, thyroid dysfunction and anxiety, because the patient did not report any abdominal pain. Due to her body habitus and the signs/symptoms of PE, uterine causes were very low on the differential. However, the uterine torsion may have been the cause of this patient’s antepartum signs and symptoms suggestive of PE, the elevated uterine artery doppler index in the second trimester, the acute change in biophysical profile, the preterm premature rupture of membranes, and the breech presentation.
Similar to adnexal torsion, uterine torsion can be asymptomatic until blood flow is severely compromised. Unfortunately, there were no ultrasonographic findings suggestive of torsion, and that diagnosis was not considered. Despite that, the mother and neonate did not sustain any serious morbidity, in part because the decision to proceed with surgery was made quickly after the finding of a non-reassuring biophysical profile.
In summation, the differential diagnosis of uterine torsion should be kept in mind in patients with the aforementioned signs and symptoms. The diagnosis is usually made intra-operatively, but can also be concluded by ultrasound or MRI. Uterine torsion may be the impetus to other obstetric complications, such as preterm rupture of membranes, or maternal complications, such as pelvic pain. For this reason, it is important to consider this diagnosis in patients with risk factors. More research needs to be done on uterine torsion in order for physicians to be aware of the proper management of this condition as well as prognosis.
Figure 1.
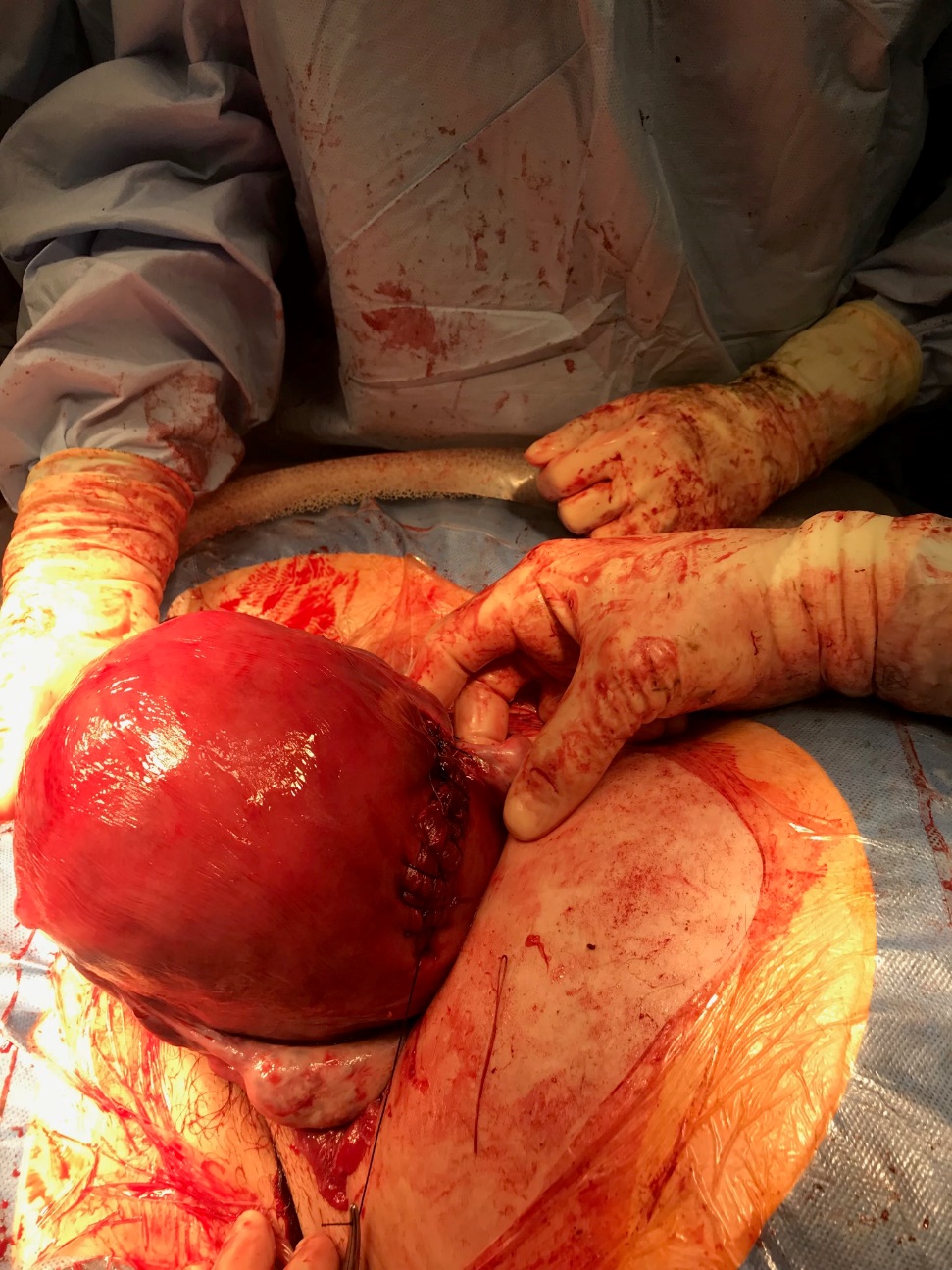
Source: Case in the hospital while working on the labor floor
Disclosure of financial support from commercial organizations: None.
Disclosure of funding from governmental organizations: none.
Uterine Torsion in an Antepartum Patient Causing Respiratory Issues
Acknowledgments: I would like to acknowledge Dr. Moshe Schwartz for pointing out this unique finding to me.