
Kalina Nikolov1, Eric Ahl2, Henrik Wagner3, 4 and Bjarne Madsen Hardig3, 4*
1Medical Faculty, Lund University, Box 117, 22100 Lund, Sweden.
2Department of Research, development and education, Helsingborg Hospital, Region Skåne, 252 23 Helsingborg, Sweden.
3Clinical Sciences, Helsingborg, Section II, Medical faculty, Lund University 251 87 Helsingborg, Sweden.
4Department of Cardiology, Helsingborg Hospital, Region Skåne. 252 23 Helsingborg, Sweden.
*Corresponding author: Bjarne Madsen Härdig, Clinical Sciences, Helsingborg, Section II, Medical faculty, Lund University 251 87 Helsingborg, Sweden.
Received date: June 05, 2023
Accepted date: June 12, 2023
Published date: June 15, 2023
Citation: Kalina Nikolov, Eric Ahl, Henrik Wagner and Bjarne Madsen Hardig (2023) “A Scoping Review on The Use of Extracorporeal Cardiopulmonary Resuscitation for Refractory Out-Of-Hospital Cardiac Arrest.” International J of Clinical Cardiology and Cardiovascular Interventions, 2(4); DOI: http;//doi.org/06.2023/1.1016.
Copyright: © 2023 Bjarne Madsen Härdig. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Aim of the review: To provide an overview of studies that have published data regarding region and population size, procedure location, team composition, inclusion and exclusion criteria, outcome parameters, and cost–benefit analyses on extracorporeal membrane oxygenation use for refractory out-of-hospital cardiac arrest.
Data sources: A structured systematic literature search of articles published prior to April 27, 2021, was performed in online databases (PubMed, EMBASE, ClinicalTrials.gov, the EU Clinical Trials Register, and Cochrane Library).
Results: Sixty-three articles were included based on predefined eligibility criteria. The included articles were published between 2011 and 2021, with the highest number of articles in 2020 and 2021 (50%). Of the 58 articles that reported data on organisational topics, 47 reported transporting the patients to the hospital for cannulation, 10 reported initiating extracorporeal cardiopulmonary resuscitation (ECPR) on-scene, and one reported doing both. The most common inclusion criterion was a lower age limit of 18 years (in 86% of the articles). Other inclusion criteria were witnessed collapse (67%) and initial ventricular fibrillation/tachycardia (43%), asystole (3%), pulseless electrical activity (5%), pulmonary embolism (2%), and signs of life during CPR (5%). The most common exclusion criterion was a do-not-resuscitate order (38%). Of the 44 studies reporting outcomes, 77% reported survival to hospital discharge and 50%, a cerebral performance category score of 1-2. Other outcome parameters were sparsely reported.
Conclusion: There is a variation in regional size, team composition, inclusion and exclusion criteria and reported outcomes. These discrepancies make it challenging to determine how to effectively use ECPR.
Extracorporeal cardiopulmonary resuscitation; Extracorporeal membrane oxygenation; Cardiac Arrest; Resuscitation; Scoping review
Introduction
Extracorporeal membrane oxygenation (ECMO) is a method in which a device acts temporarily as the heart and lungs to oxygenate and circulate blood when these functions are compromised. Blood bypasses the cardiopulmonary system moving from the femoral vein into a cannula to be oxygenated by the device and then pumped back into a second artery or vein. One of many conditions for which ECMO can be used is refractory cardiac arrest (CA), as a supplement to standard cardiopulmonary resuscitation (CPR), called extracorporeal cardiopulmonary resuscitation (ECPR).1 Refractory CA is defined as a lack of return of spontaneous circulation despite advanced CPR according to guideline recommendations.2,3 ECPR can instantly restore circulation and the crucial perfusion to the brain, allowing more time for treatment of the underlying cause of the arrest; hence, it has the potential to improve survival and neurological outcome for patients with refractory CA.4 European Resuscitation Council and American Heart Association guidelines state that it could be considered for selected patients with CA when conventional advanced life support measures fail; they recommend it for potentially reversible causes or to facilitate specific interventions, such as coronary angiography, percutaneous coronary intervention, pulmonary thrombectomy for massive pulmonary embolism, and rewarming after hypothermic CA in settings in which it can be implemented.2,3 Centres around the world have formed their own protocols for the use of ECPR for refractory out-of-hospital CA (OHCA).4–9 Most programmes have inclusion criteria such as age, comorbidities, bystander CPR, estimated distance to hospital, and time to ECMO cannulation.6 The location where the cannulation is performed varies. While some programmes institute the ECMO device at the site of the arrest, some cannulate inside a specialised ECMO-equipped vehicle, and others transport the patient to a waiting team in the hospital, each resulting in its own unique personnel composition and total programme cost.10,11
While there have been many observational studies showing promising results for ECPR compared to standard advanced cardiac life support, the first randomised controlled trial was published in 2020.4 Research on emergency treatment for OHCA is of interest because globally, the survival rate remains low, with only 1 in 10 individuals surviving hospital discharge.12–13 In 2017, Skåne County, Sweden, where this scoping review was conducted, had a survival rate of 11.5%;14 however, there is no local ECPR programme despite 1200 OHCAs per year and a population of 1.4 million.15
The objectives of this scoping review were to provide an overview and systematically evaluate reported data from articles regarding region and population size, procedure location, team composition, adherence to inclusion and exclusion criteria, outcome parameters reported and cost–benefit analyses on the use of ECPR for refractory OHCA.
Methods
A structured systematic literature search was developed by an experienced senior research librarian. A search strategy according to a predefined protocol based on the PRISMA 2020 Checklist16 was used for the master programme. Registration of this analysis was deferred owing to the lack of systematic review or meta-analytic data. The study did not require institutional review board approval as human participant protected health information was not used.
Eligibility criteria
Studies eligible for inclusion included randomised controlled trials, observational, case reports, case series, pilots, protocols, feasibility studies and cost analyses that evaluated ECPR for OHCA and were published before April 27, 2021. Studies that included children (age <18 years), animals, or other ECMO use such as post-surgery use, organ transplantation, non-cardiac aetiology, coronavirus disease 2019, or cardiogenic shock, were excluded.
Information Sources
The following databases were searched according to the predefined eligible criteria: PubMed, EMBASE, ClinicalTrials.gov, the European Union Clinical Trials Register, and Cochrane Library, which included articles that were published prior to April 27, 2021.
Search
The full electronic search strategy for the databases used is presented in Supplementary Material A1. In PubMed and Clinical Trials Register, there were two search strategies used, whereas in the Cochrane library and EMBASE database, one search strategy was used. No filters were applied in any of the databases or searches done. The reference list of the included articles was screened for additional studies. Studies with no full text available or in languages other than English were excluded. For organisations that first published a study protocol and later published the completed trial based on the same protocol, only the completed trial was included. The included articles were compiled in an Excel worksheet (Microsoft, Redmond, Washington, United States).
Selection of sources of evidence
The selection of sources was performed according to the predefined protocol by two authors, and no disagreement regarding study selection and data extraction was noted.
Data charting process
A data charting form was jointly developed by two of the authors to determine which variables to extract. The two authors independently charted the data and discussed any disagreement regarding the charting.
Data items
The data items extracted were population, city, country, patient eligibility criteria, defined inclusion and exclusion criteria, team composition, procedure location, study period, study design, and cost–benefit analyses. For studies presenting results, the demographic data extracted included the number of patients that received ECMO, age, and sex. Moreover, factors known to be important for survival following a CA, such as if the CA was witnessed or not, if the patient had received bystander CPR, if the patient had an initial shockable rhythm or not, and how outcomes such as survival and neurological outcomes were measured at hospital discharge, 1 month, 3 months, 6 months or 1 and 5 years, were reported.
Critical appraisal of individual sources of evidence
All articles that met the objectives of this scoping review and provided an overview of data regarding region- and population size, procedure location, team composition, inclusion and exclusion criteria, outcome parameters reported, and cost-benefit analyses when using ECMO for refractory OHCA were included.
Statistics and synthesis of results
Furthermore, data regarding region and population size, procedure location, team composition, and inclusion factors, were compared to the outcome reported in the studies. However, no statistical comparisons could be made, as there was a large variation in the evaluated parameters as well as how outcomes were reported. Data are presented as numbers and percentages (N (%)), average and standard deviation or median and min-max as appropriate for each set of data.
Results
Search results and selection of articles
The initial search yielded a total of 2,809 articles, which were weeded down to 2,787 after the removal of duplicated articles. Overall, 392 abstracts were read and 163 full-text articles on ECPR for refractory OHCA were found. Of these articles, 61 were eligible for inclusion according to the predefined criteria. Two more articles were found during the reference list screening, resulting in 63 eligible articles 4-6, 10-11,17-72 included in this scoping review (Figure 1).
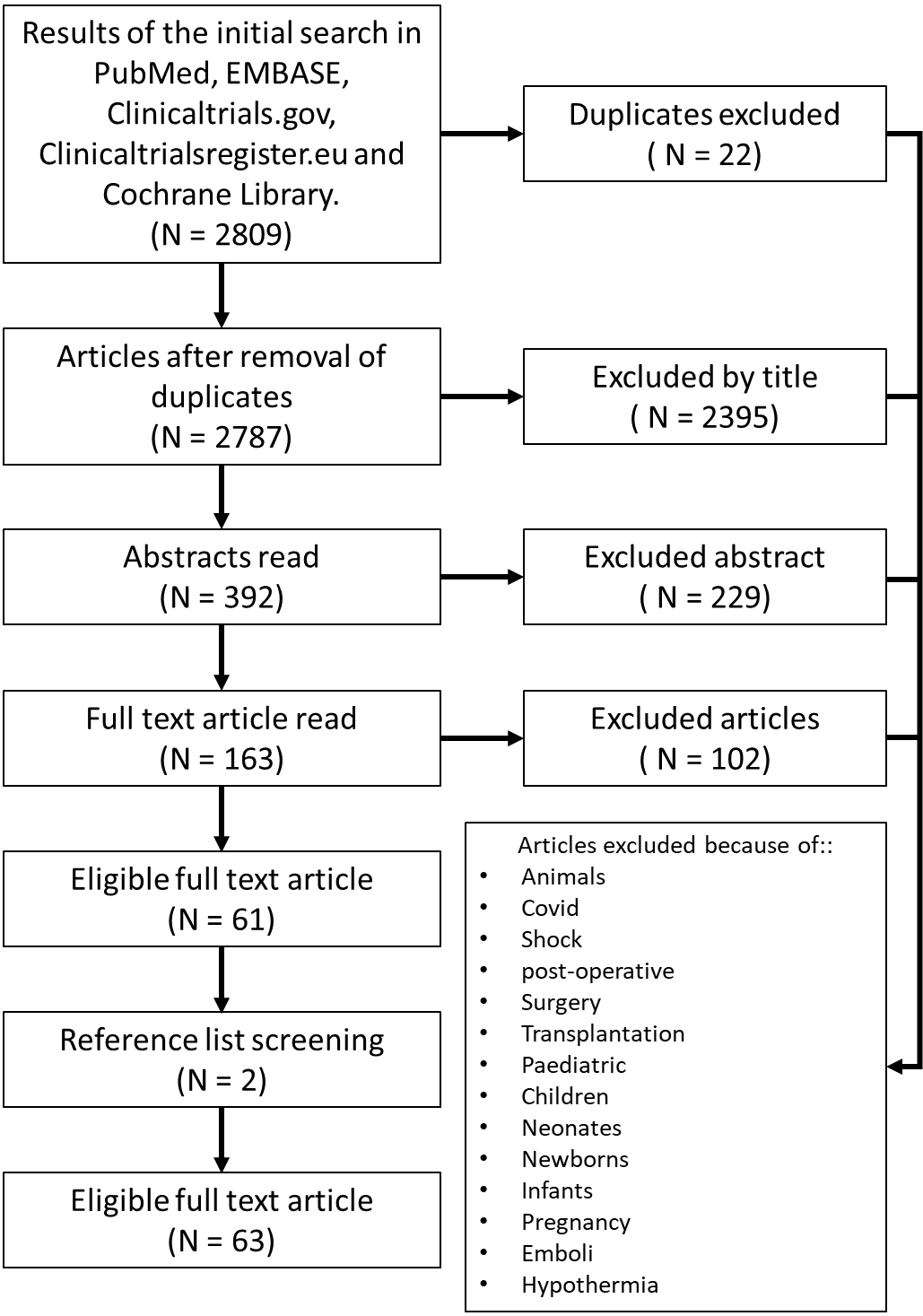
Figure 1. Flowchart for the search strategy, selection, and exclusion of articles.
Article types and publication years
The included articles were published between 2011 and 2021 with the highest number of articles published between 2020 and 2021 (50%). The most common type of studies were observational retrospective cohort studies (48%) (Table 1). Figure 2A shows the survival to hospital discharge in relation to publication year, which shows that the highest median survival was found in articles published in 2020 and 2021; however, there was a large variation in survival to hospital discharge when comparing studies regarding publication year (Figure 2A).
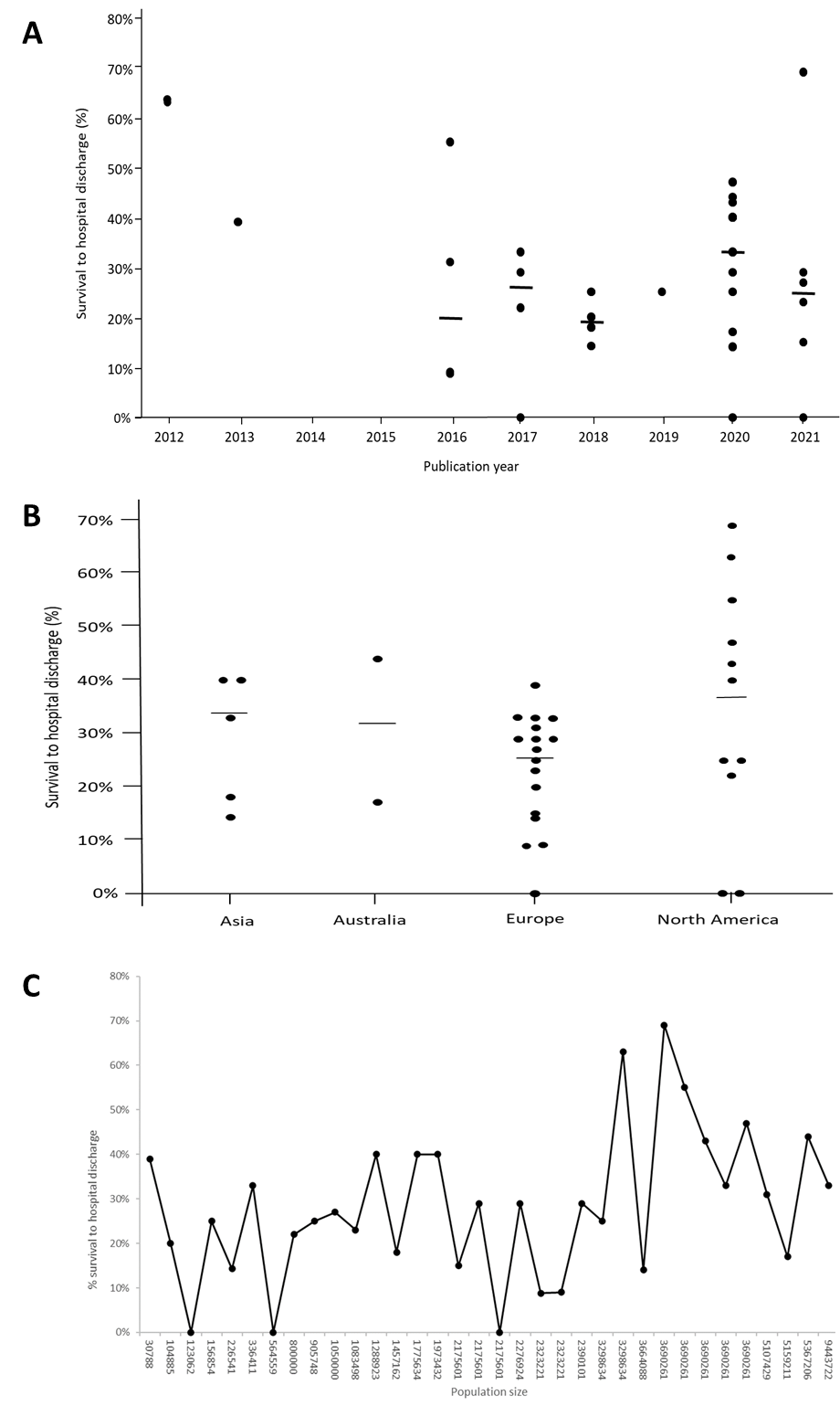
Figure 2. A: Shows the median (Max-Min) survival to hospital discharge in relation to publication year. B: Shows the median (Max-Min) survival to hospital discharge for the 4 continents that had included patients. C: Shows the median survival to hospital discharge in relation to the population size the organisation is covering in their system.
Description of regions and number of times ECPR was performed.
Included articles were from four continents (Europe, North America, Asia, and Australia). The highest number of articles were from Europe (50%) followed by North America (24%). In Europe, most articles came from Germany (19%), while in North America, most articles came from the USA (23%). The highest median survival rate was noted in the studies performed in North America (37%) followed by Asia (33%), Australia (31%), and Europe (25%) but varied substantially in all regions (Figure 2B). The ECMO teams covered different population sizes and the median population size was 2,323,221 (ranging from 30,788 and 19,303,000). The population size of the area where the ECMO teams work does not seem to influence survival to hospital discharge as the variation in survival rate varies greatly independently of population size (Figure 2C). The number of patients treated with ECPR in the study period reported in the articles varied between 0.1 and 11.6 patients per month, with a median of 0.7 patients per month. Figure 3A shows that there is a weak correlation (R2 value: 0.0307) between survival to hospital discharge and the number of patients treated per month.
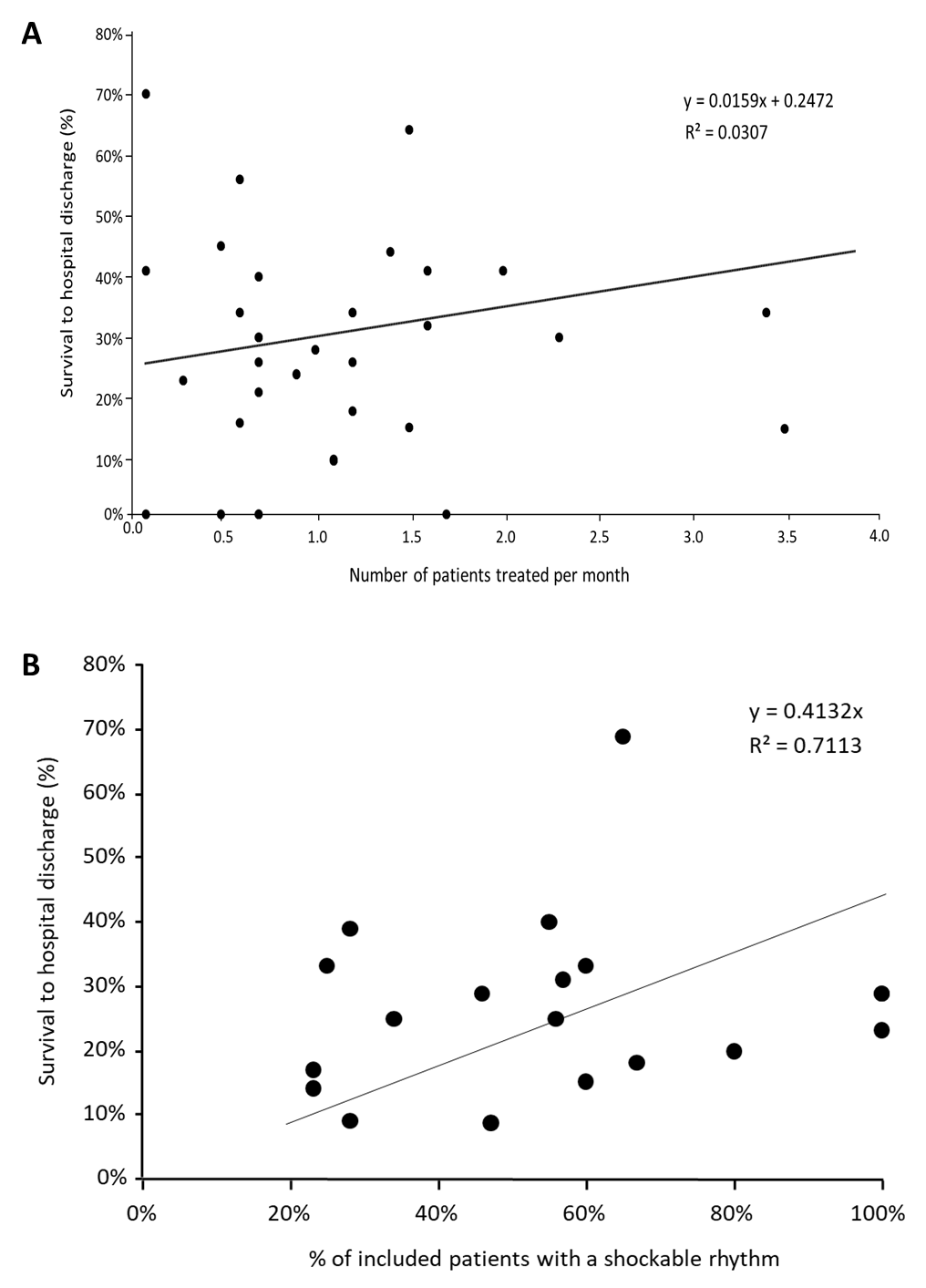
Figure 3. A: Shows the correlation between survival to hospital discharge and the number of patients treated per month in the individual studies. B: Shows the correlation between survival to hospital discharge and the number of patients that were included with an initial rhythm of ventricular fibrillation or ventricular tachycardia.
Team composition and location of the procedure
The number of members in the ECPR teams varied between two and eight persons, where the most common number was three (41%). The professions involved in the team also varied: physicians (not specified), emergency physicians, intensivists, cardiologists, and thoracic surgeons. Nurses’ special competence was not specified in any of the articles. The other professions involved were paramedics, perfusionists, pharmacists, technicians, and respiratory therapists. Most of the teams instituted the ECMO in the hospital (81%), whereas 17% used a mobile team and one did both (2%). When the ECMO was instituted in the hospital, the procedure was performed most often in a catheterisation laboratory (47%); however, general wards were also used, probably to shorten the time to the start of the ECMO. The mobile teams instituted the ECMO at a scene or in an ambulance (Figure 4A and B). The relationship between survival to hospital discharge and team composition is shown in Figure 5, which shows a median survival rate between 18% (seven personnel teams) and 33% (three and five personnel teams). The median survival rate to hospital discharge in those studies where the ECMO was instituted in the hospital was 29% (0-69%) compared with the 15% (0-49%) observed if the ECMO was instituted outside the hospital as shown in Figure 6.
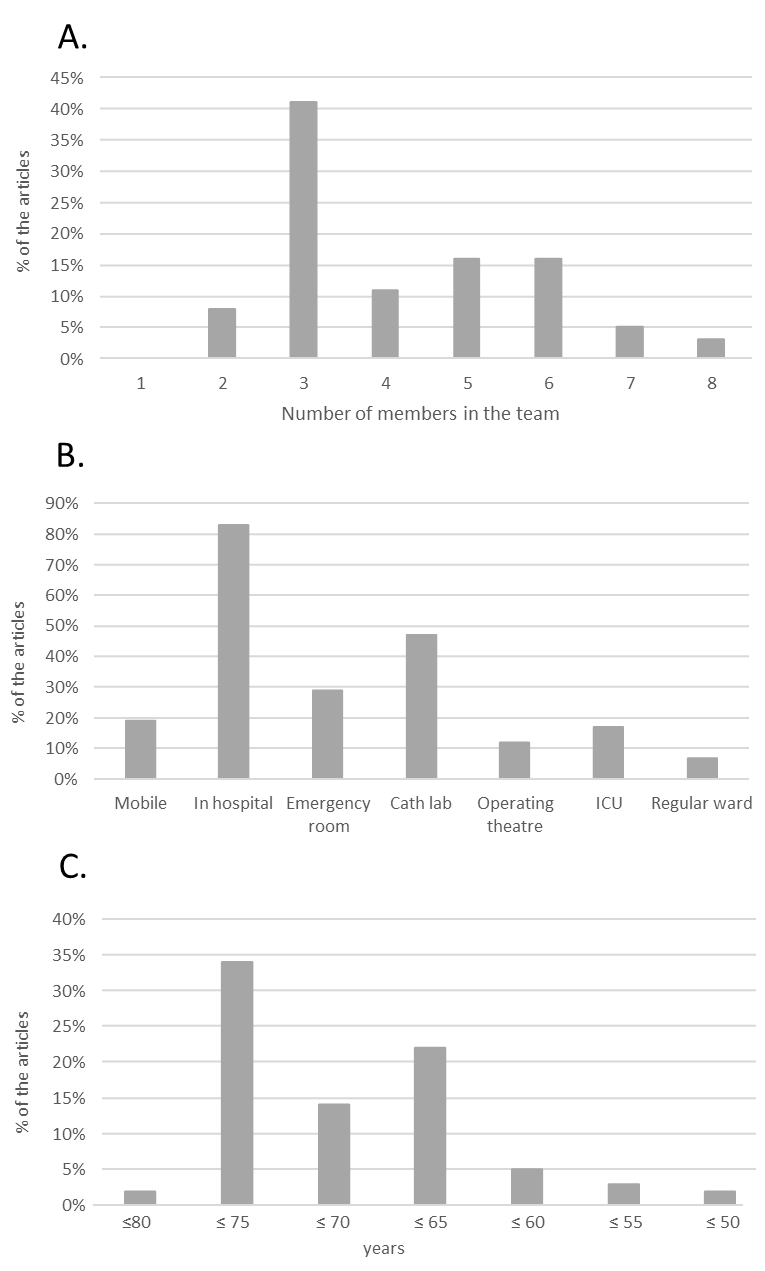
Figure 4. A: Shows the number of members in the different teams. B: Shows the places where the different organisations institute the ECMO device. C: Shows distribution of age limit for inclusion in the different studies.
Inclusion and exclusion criteria
The most common inclusion criterion was a lower age limit of 18 years (present in 86% of the studies), followed by an upper age limit (83%). However, the upper age limit ranged between 50 and 80 years with a median of 70 years (Figure 4C). In studies that included patients (and reported the age of the included patients), only two diverged from the stated age criteria (Appendix A2). Other inclusion criteria were witnessed collapse (67%), initial shockable rhythm (43%), asystole (3%), pulseless electrical activity (5%), pulmonary embolism (2%), and signs of life during CPR (5%). Of the studies that reported witnessed collapse as an inclusion criterion, nine studies did not report the number of witnessed arrests that was included, nine reported that they included 100% of the arrests that were witnessed, and nine studies, that they included between 57-93% of the arrests that were witnessed; therefore, only 9 studies strictly used witnessed collapse as an inclusion criterion. The definition of a refractory CA varied between the articles using different denominators, from the number of shocks to no return of spontaneous circulation after a pre-specified time of advanced life support (Table 2). No-flow time (defined as time without chest compression) ranged between 0 and 20 min, and low-flow time (defined as the time from starting chest compressions to ECMO utilisation) ranged between 30 and 150 min. In studies where ECMO was instituted in the hospital, 17% had a transport time limit (≤30 min (10-40 min)). Of the studies (n = 13) that reported inclusion criteria for both no-flow and low-flow time and included patients, only five studies followed their stipulated times. These studies had a combined no-flow and low-flow time of more than 90 min. In the remaining studies, the no-flow and low-flow time exceeded the time stated in their inclusion criteria (Appendix A3). A small number of articles had blood parameters and physiological measurements as inclusion criteria (Table 2). The most commonly reported exclusion criteria were a do-not-resuscitate order (38%) followed by major comorbidity (36%), terminal illness (31%), and trauma/bleeding (29%). Details of the exclusion criteria are presented in Table 2.
Appendix A2.
|
Age Min |
Age Max |
Age Mean |
Age SD |
Age Median |
Age Min |
Age Max |
|
18 |
55 |
44 |
11 |
NaN |
NaN |
NaN |
|
18 |
55 |
NaN |
NaN |
44 |
21 |
71 |
|
18 |
60 |
NaN |
NaN |
46 |
38 |
53 |
|
18 |
60 |
NaN |
NaN |
47 |
32 |
53 |
|
18 |
65 |
66 |
12 |
NaN |
NaN |
NaN |
|
18 |
65 |
NaN |
NaN |
53 |
45 |
60 |
|
18 |
65 |
NaN |
NaN |
50 |
43 |
56 |
|
18 |
65 |
NaN |
NaN |
46 |
35 |
61 |
|
18 |
65 |
NaN |
NaN |
50 |
43 |
56 |
|
18 |
65 |
56 |
14 |
NaN |
NaN |
NaN |
|
18 |
70 |
57 |
37 |
NaN |
NaN |
65 |
|
18 |
70 |
51 |
21 |
NaN |
NaN |
NaN |
|
18 |
70 |
47 |
16 |
NaN |
NaN |
NaN |
|
18 |
70 |
62 |
8 |
NaN |
NaN |
NaN |
|
18 |
75 |
44 |
14 |
NaN |
NaN |
NaN |
|
18 |
75 |
57 |
2 |
NaN |
NaN |
NaN |
|
18 |
75 |
57 |
13 |
NaN |
NaN |
NaN |
|
18 |
75 |
56 |
14 |
NaN |
NaN |
NaN |
|
18 |
75 |
56 |
NaN |
NaN |
NaN |
NaN |
|
18 |
75 |
NaN |
NaN |
62 |
52 |
66 |
|
18 |
75 |
NaN |
NaN |
60 |
50 |
68 |
|
18 |
75 |
53 |
12 |
NaN |
NaN |
NaN |
|
18 |
75 |
53 |
13 |
NaN |
NaN |
NaN |
|
18 |
75 |
55 |
14 |
NaN |
NaN |
NaN |
|
18 |
75 |
57 |
19 |
NaN |
NaN |
NaN |
|
18 |
75 |
NaN |
NaN |
56 |
43 |
65 |
|
18 |
75 |
NaN |
NaN |
60 |
55 |
67 |
|
18 |
75 |
52 |
12 |
NaN |
NaN |
NaN |
|
18 |
75 |
NaN |
NaN |
54 |
47 |
62 |
|
18 |
75 |
NaN |
NaN |
59 |
36 |
73 |
|
18 |
75 |
50 |
11 |
NaN |
NaN |
NaN |
|
18 |
75 |
NaN |
NaN |
65 |
NaN |
NaN |
|
18 |
75 |
NaN |
NaN |
56 |
45 |
66 |
|
18 |
80 |
NaN |
NaN |
66 |
46 |
75 |
|
18 |
NaN |
NaN |
NaN |
56 |
43 |
70 |
|
18 |
NaN |
NaN |
NaN |
62 |
49 |
76 |
|
SD = Standard deviation |
||||||
Appendix 3A.
|
NFT+ LFT (min) |
Time to ECMO Mean |
Time to ECMO SD |
Time to ECMO Median |
Time to ECMO Min |
Time to ECMO Max |
|
35 |
49 |
17 |
|||
|
45 |
89 |
65 |
107 |
||
|
60 |
87 |
27 |
|||
|
60 |
60 |
58 |
85 |
||
|
60 |
51 |
22 |
70 |
||
|
60 |
62 |
50 |
75 |
||
|
65 |
51 |
37 |
80 |
||
|
90 |
89 |
73 |
111 |
||
|
95 |
60 |
45 |
80 |
||
|
105 |
84 |
21 |
|||
|
110 |
50 |
27 |
95 |
||
|
150 |
115 |
84 |
140 |
||
|
150 |
84 |
55 |
122 |
||
|
NFT = No flow time, LFT = Low-flow time, ECMO = Extra corporeal membrane oxygenation, SD = standard deviation.
|
|||||
Figure 5. Shows the median (Max-Min) survival to hospital discharge in relation to the number of personnel reported to be involved in the procedure in the different articles.
|
Defined inclusion criteria |
Articles (n (%)) |
|
Upper age limit |
48 (83%) |
|
Lower age limit ≥18 |
50 (86%) |
|
Bystander CPR |
26 (45%) |
|
Witnessed CA |
39 (67%) |
|
Initial shockable rhythm |
25 (43%) |
|
Asystole |
2 (3%) |
|
PEA |
3 (5%) |
|
No-ROSC criteria |
11 (16%) |
|
No-ROSC after 2 shocks |
2 (3%) |
|
No-ROSC after 3 shocks |
3 (5%) |
|
No-ROSC after 5 min of CPR |
1 (2%) |
|
No-ROSC after 10 min of CPR |
1 (2%) |
|
No-ROSC after 15 min of CPR |
3 (5%) |
|
No-ROSC after 20 min of CPR |
1 (2%) |
|
Pulmonary Embolism |
1 (2%) |
|
Signs of life during CPR |
3 (5%) |
|
No-flow time limit |
16 (28%) |
|
Low-flow time limit |
9 (16%) |
|
CA-to-ECMO time limit |
24 (34%) |
|
Transport time limit |
10 (17%) |
|
Blood parameters prior to ECMO initiation |
|
|
Arterial-Lactate level (mg/dL) |
2 (3%) |
|
Arterial-Ph level |
2 (3%) |
|
Systolic BP (mmHg) |
1 (2%) |
|
Arterial-PO2 (mmHg) |
1 (2%) |
|
End-tidal CO2 (mmHg) |
11 (19%) |
|
Exclusion criteria |
|
|
Trauma/Bleeding |
17 (29%) |
|
Terminal illness |
18 (31%) |
|
Major comorbidities |
21 (36%) |
|
DNR |
22 (38%) |
|
Neurological deficits |
15 (26%) |
|
Malignancy |
13 (22%) |
|
Pregnancy |
10 (17%) |
|
CPR = Cardio- Pulmonary Resuscitation, PEA = Pulseless Electrical Activity, ROSC = Return of Spontaneous Circulation, CA = Cardiac Arrest, ECMO = Extra corporeal membrane oxygenation, BP = Blood Pressure, DNR = Do Not Resuscitate |
|
Table 2. The inclusion and exclusion criteria of the included articles.
Background and outcome parameters for studies that had included patients.
Forty-four studies included patients with a median number of 44 (1–320) patients. Background parameters reported, such as age (100%) sex (89%), shockable rhythm (68%), time to ECMO (66%), witnessed arrest (61%), and bystander CPR (41%), varied between the studies (Table 3). There was a positive correlation between the number of patients with a shockable rhythm and survival to hospital discharge (R2 = 0.7113) as shown in Figure 3B. The other rhythms included were too few; hence, it was difficult to analyse their correlation to survival. The most common outcome parameter reported in the studies was survival to hospital discharge (77%). The median number for survival to hospital discharge in those studies was 28% (0-69%); six (14%) of the studies reported 1-month survival (22% (13-28%)) and two studies (5%) reported 1-year survival (37% and 69%). Twenty-two studies (50%) reported neurologic outcomes using the Cerebral Performance Category (CPC) scale (CPC 1-2) and reported a median CPC 1-2 of 20% (4-65%); however, only a few studies reported the other categories of the CPC scale (Table 3).
|
Background and outcome parameters (n=44) |
Articles (n (%)) |
Median (Min-Max) |
|
Number of patients |
44 (100%) |
40 (1-320) |
|
Age presented as Median (Min-Max) |
21 (48%) |
56 (19-76) |
|
Age presented as Mean (SD) |
20 (45 %) |
56 (2-21) |
|
Age presented as Mean only |
3 (7%) |
65 (56-66) |
|
Gender (males) |
39 (89%) |
76% (57-100%) |
|
Bystander CPR |
18 (41%) |
63% (21-100%) |
|
Shockable rhythm |
30 (68%) |
58% (23-100%) |
|
Witnessed arrest |
27 (61%) |
100% (57-100%) |
|
Time to ECMO presented as Median (Min-Max) |
14 (48%) |
60 (21-192) |
|
Time to ECMO presented as Mean (SD) |
10 (34%) |
84 (17-56) |
|
Time to ECMO presented as mean only |
5 (17%) |
41 (27-84) |
|
Outcome (N= 44) |
Articles (n (%)) |
Median (Min-Max) |
|
Survival to hospital discharge |
34 (77%) |
28% (0-69%) |
|
1-month survival |
6 (14%) |
22% (13-28%) |
|
3-month survival |
2 (5%) |
43% (39-46%) |
|
6-month survival |
3 (7%) |
43% (37-69%) |
|
1-year survival |
2 (5%) |
43% (37-69%) |
|
5-years survival |
1 (2.5%) |
66% |
|
CPC-score 1-2 |
22 (50%) |
20% (4-65%) |
|
CPC-score 3-4 |
5 (11%) |
4% (2-22%) |
|
CPC-score 1 |
7 (16%) |
19% (3-33%) |
|
CPC-score 2 |
5 (11%) |
5% (3-22%) |
|
CPC-score 3 |
4 (9%) |
2% (0-3%) |
|
CPC-score 4 |
4 (9%) |
3% (3-5%) |
|
CPC-score 5 |
5 (11%) |
86% (40-91%) |
|
CPR = Cardio- Pulmonary Resuscitation, ECMO = Extra Corporeal Membrane Oxygenation, CPC = Cerebral performance category Table 3. Background information and outcome parameters for 44 of the studies that had included patients. |
||
Cost–benefit analysis
We found four studies that analysed the cost-effectiveness of ECPR.69–72 One of the studies noted that ECPR was “highly cost-effective” if the patient presented with a shockable rhythm. Cost per life saved was reported as half of the cost for those with ventricular fibrillation or ventricular tachycardia compared to those with asystole or pulseless electrical activity.69 One study concluded that ECPR has a favourable cost-effectiveness threshold.70 Another study concluded that the treatment was cost-effective when compared to the cost of heart transplantation for end-stage heart failure,71 and the fourth found that ECPR is economically justifiable in a transplant centre.72 However, only one of these studies included the pre-hospital cost in their analysis.72
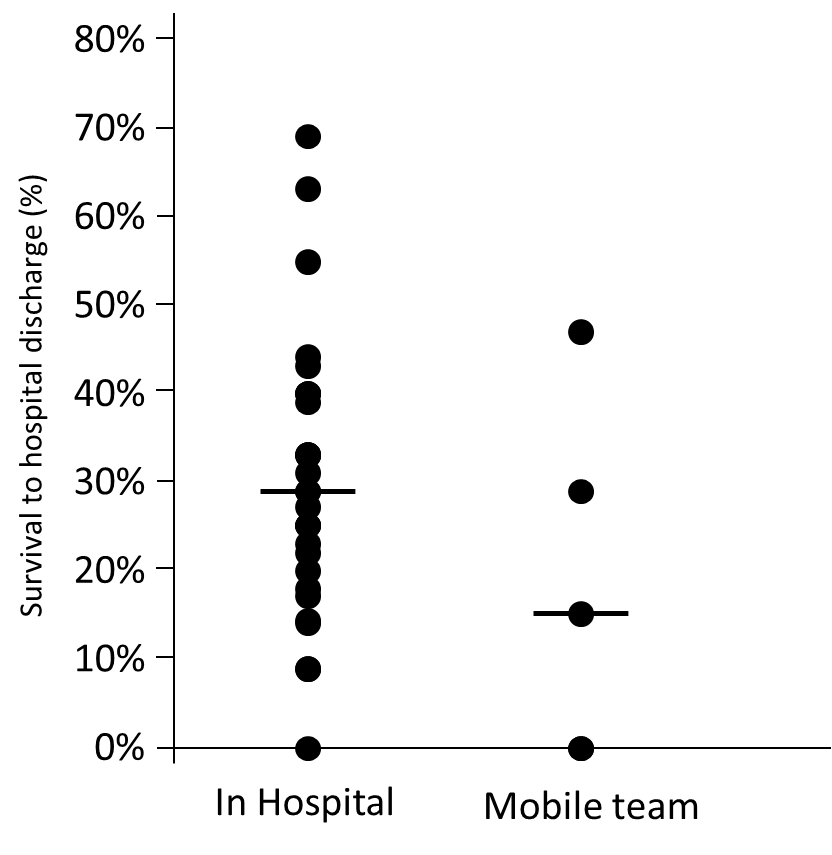
Figure 6. Shows the median (Max-Min) survival to hospital discharge comparing those studies where the ECMO was instituted inside the hospital compared to those using a mobile team.
Discussion
According to the objectives of this scoping review, 63 eligible articles were included. The included articles were published between 2011 and 2021 with the highest number of articles in 2020 and 2021 (50%). We observed an improvement in the survival rate per year, as the highest median survival rates were found in articles published in 2020 and 2021. Other findings were that organisations vary greatly in regional size, team composition, and inclusion and exclusion criteria. There is also variation in the location where the procedure was performed and how the outcomes are reported.
One of the focuses of this review was to analyse the team composition, as it is an important aspect to understand how ECPR organisations produce optimal workflow; however, only a few articles described it. There was one article with otherwise detailed inclusion and exclusion criteria that did not report on the composition of the personnel involved, being therefore excluded from the review. Since team compositions ranged from two to eight members for the same procedure, there might be room for improving efficiency and lowering the programme’s costs.28, 29 The extracted personnel statistics would have been of more interest if there were studies on cost efficiency comparing personnel costs – which is worth investigating in the future when more studies on the costs for ECPR for refractory CA have been published.69-72 Regarding team composition, some studies found it important to mention the specialties of the physicians but omitted the specialties of the nurses, while others even included the driver of the mobile programme.20,29 For example, it is unlikely that, in a study that mentioned only two physicians, help from nurses or other professionals was not required.26
Given that the treatment is time-dependent, implementing mobile on-scene organisations might make more sense to improve survival. Installing an ECMO device inside a vehicle presents a whole new set of logistics and costs, but yields promising results.23,50 We noted that the survival to hospital discharge was lower in articles that reported using a mobile team. Nevertheless, perhaps this time-effective approach will be pursued by more programmes once further research in this area is available.10, 50
Koen et al.73 identified witnessed CA as an insignificant eligibility criterion; however, this literature review showed that it was the second most common criterion (67%). Koen et al. also noted that a no-flow time of less than 5 min was the most important inclusion criterion, which was only found as such in 28% of the analysed articles. We also noted that these time frames were exceeded in six out of 10 studies. Thus, better adherence to no-flow and low-flow time could improve the chances of survival. We also noted that the higher the number of patients with ventricular fibrillation or ventricular tachycardia that were included, the higher the chance of survival to hospital discharge observed (Figure 3B). This is in line with the results in the cost-benefit analysis that observed that for patients with a shockable rhythm (ventricular fibrillation or ventricular tachycardia), cost per life saved was reported as half of the cost compared to those with asystole or pulseless electrical activity.69 However, a recent study showed that patients with asystole could be eligible for inclusion if they were witnessed, received bystander CPR, and had signs of life or a pupil diameter size <5 mm at hospital arrival;74 therefore, the inclusion criteria for this group might differ compared to those for patients with ventricular fibrillation or ventricular tachycardia.
Bystander CPR was the fourth most common inclusion criterion in this analysis but was not noted as an important factor by Twohig et al. in their literature review.75 However, most current organisations did not have eligibility criteria in line with or did not report that they followed the best available evidence. This may be solved using randomised trials with pre-specified eligibility criteria; however, only one randomised controlled trial had ever been completed and reported when this analysis was performed.4 The ECPR group in the randomised Minneapolis trial reached a respectable 43% survival rate,4 which was higher compared to those in most of the earlier observational studies included in this literature review (28% (0-69%)). Supported by Twohig et al., the Minneapolis group had an initial shockable rhythm and did not have bystander CPR or no-flow time as criteria. On the other hand, they only included witnessed CA, opposing the suggestion from Twohig et al.4,75 Patient eligibility criteria – who is suitable for further resuscitation attempts and who is not – is an ethical question. Some programmes name very few exclusion criteria; thus, giving even the very frail a chance,19 whereas others have an extensive and narrow list that makes only a small fraction of an initially large OHCA population considered as ECPR candidates.45 Presently, a patient might not meet the treatment criteria in one region, but an identical patient in a different region will, and will have the chance to survive. With a recent study showing that patients with asystole could be eligible for inclusion, further research on how to best select patients for ECPR may be required.74
Outcome reporting of CA studies has been defined by the Ustein template,76 which should be used when reporting OHCA studies. In the analysed studies that reported outcome data, significantly few followed the template regarding background parameters and reporting outcomes. In 77% of the studies, survival to hospital discharge was reported, and only 14% reported 1-month survival. Additionally, only 50% reported neurological outcomes and CPC score for the patients, and the score range was wide (CPC 1-2: 4%-65%). When not using standardised reporting after OHCA, it is difficult to judge the results of this highly advanced treatment regimen. Several of the analysed studies state that there is a need for more randomised studies in this field. However, randomised studies could bring another ethical dilemma to the field of emergency medicine research. On one hand, there is a need for randomised trials as is pointed out by many studies.20,48,59 On the other hand, there are already regions that have implemented ECPR based on the notion that it saves lives and is recommended in the current guidelines for advanced life support.3,4 When ECPR has already saved a portion of patients, it might be difficult to randomly allot some people to standard advanced cardiac life support only, as the knowledge of doing so could be thought to be unethical.77 In the randomised study in Minneapolis, the safety monitoring board of the trial had the study terminated early after seeing overwhelmingly improved survival with ECPR.4 One argument for the difficulty in performing randomised controlled trials with informed consent in the field of emergency medicine is the limited time required for these time-dependent treatments. However, there are ways of dealing with consent in emergency medicine studies: the PARAMEDIC 2 study78 informed citizens of the public so that those who did not want to be enrolled in the PARAMEDIC 2 trial had the option of requesting a stainless-steel bracelet that had the words “NO STUDY” engraved on it. These could then be noted by the personnel when there was a CA, and the patient could be given normal treatment.81 This strategy could also be used for ECPR research.
The size of the region and population do not seem to be a prerequisite for having an ECPR organisation, as the smallest population was 30,788.61 A retrospective Danish study had a population and coverage area like that of Skåne County, which has 1.4 million habitants with an area of 11,000 km2.61 The Danish programme covered slightly fewer people (1.3 million habitants) but a larger area of 13,000 km2 and reported a survival rate of 33% for refractory CA treated with ECPR.61 Scale-wise, the two consider it feasible to start a local OHCA ECMO programme in Skåne County and other regions comparable in size to those analysed in this review. The number of patients treated per month varied greatly among the analysed studies, and this variance could be a factor that contributed to the good outcomes. However, we could not show that this was the case for other studies (Figure 3A), where a low correlation was observed.
Limitations
In this scoping review, we analysed articles that contained data for the predefined criteria chosen for this purpose, namely region and population size, cost–benefit analyses, inclusion and exclusion criteria adherence, procedure location, team composition, and outcome parameters reported. However, the study was not able to clarify which system or organisation works best. This could not be performed owing to the heterogeneous article types included in the study. This also means that specific data that could balance some systems’ results of improvement were excluded; however, this is the nature of using predefined criteria for inclusion that is required when performing a systemic review. Another notable limitation of this review was the exclusion of articles that mentioned cardiogenic shock, myocardial infarction, pulmonary embolism, and hypothermia, which led to the exclusion of ECMO programmes that treated several aetiologies besides patients with refractory CA, as these programmes did not have separate results for refractory CA. The same applies to programmes including children. Furthermore, it may be of interest to separately investigate organisations treating a wide selection of aetiologies instead of refractory CA only. By using the same team and device for several causes, the cost-effectiveness could be considerably better than using them for only ECPR for refractory OHCA.
Conclusion
Within the published literature used for this scoping review, organisations vary greatly in regional size, team composition, inclusion, and exclusion criteria, where the procedure is performed, and how the outcomes are reported. These discrepancies make it challenging to determine how to use this strategy in the most effective manner and will affect matters such as cost-benefit analyses of ECPR for refractory OHCA. Despite an increasing amount of research published in the last few years, there remains a need for unified inclusion and exclusion criteria, outcome reporting, and a better understanding of what the optimal team composition and location for the procedure are.
Appendix A1
This document shows all search strings used for the article: A scoping review on how hospitals around the world are organised regarding the use of extracorporeal cardiopulmonary resuscitation for refractory out-of-hospital cardiac arrest.
Appendix A2
This document shows the adherence to the stated age criteria for inclusion in the different studies included in this scoping review. Cells marked with green indicates studies that followed their stated inclusion criteria and red marked cell indicates studies which did not.
Appendix A3
This document shows the adherents to the stated no-flow and low-flow times for inclusion in the different studies included in this scoping review. Cells marked with green indicate studies that followed their stated inclusion criteria and red-marked cells indicate studies which did not.
The search string used for the PubMed search.
("heart arrest"[MeSH Terms] OR "heart arrest"[Text Word] OR "cardiac arrest"[Text Word] OR "myocardial infarction"[Text Word] OR "cardiopulmonary arrest"[Text Word] OR "ventricular fibrillation"[MeSH Terms] OR "ventricular fibrillation"[Text Word] OR "tachycardia, ventricular"[MeSH Terms] OR "ventricular tachycardia"[Text Word]) AND ("ecmo"[Text Word] OR "extracorporeal membrane oxygenation"[MeSH Terms] OR "extracorporeal membrane oxygenation"[Text Word] OR "ECPR"[Text Word] OR "extracorporeal cardiopulmonary resuscitation"[Text Word] OR "ECLS"[Text Word] OR "extracorporeal life support"[Text Word]) NOT ("pigs"[Text Word] OR "covid"[Text Word] OR "shock"[Title] OR "post-operative"[Text Word] OR "surg*"[Title] OR "transplant*"[Title] OR "pediatric"[Title] OR "paediatric"[Title] OR "child*"[Title] OR "neonat*"[Title] OR "newborn"[Title] OR "infan*"[Title] OR "pregnan*"[Title] OR "emboli*"[Title] OR "hypotherm*"[Title])
The search string used for the PubMed cost-focused search.
("heart arrest"[MeSH Terms] OR "heart arrest"[Text Word] OR "cardiac arrest"[Text Word] OR "myocardial infarction"[Text Word] OR "cardiopulmonary arrest"[Text Word] OR "ventricular fibrillation"[MeSH Terms] OR "ventricular fibrillation"[Text Word] OR "tachycardia, ventricular"[MeSH Terms] OR "ventricular tachycardia"[Text Word]) AND ("ecmo"[Text Word] OR "extracorporeal membrane oxygenation"[MeSH Terms] OR "extracorporeal membrane oxygenation"[Text Word] OR "ECPR"[Text Word] OR "extracorporeal cardiopulmonary resuscitation"[Text Word] OR "ECLS"[Text Word] OR "extracorporeal life support"[Text Word]) NOT ("pigs"[Text Word] OR "covid"[Text Word] OR "shock"[Title] OR "post-operative"[Text Word] OR "surg*"[Title] OR "transplant*"[Title] OR "pediatric"[Title] OR "paediatric"[Title] OR "child*"[Title] OR "neonat*"[Title] OR "newborn"[Title] OR "infan*"[Title] OR "pregnan*"[Title] OR "emboli*"[Title] OR "hypotherm*"[Title]) AND (econom*[text word] OR cost*[text word] OR expens*[text word] OR financ*[text word])
The search string used for the Clinical Trials Register search.
(Extracorporeal Membrane Oxygenation) OR (ECMO) OR (ECPR) OR (Extracorporeal
cardiopulmonary resuscitation) OR (ECLS) OR (Extracorporeal Life Support) AND (heart arrest) OR (cardiac arrest) OR (myocardial infarction) OR (cardiopulmonary arrest) OR (ventricular fibrillation) OR (ventricular tachycardia)
The search string used for the Clinical Trials search.
Condition:(heart arrest) OR (cardiac arrest) OR (myocardial infarction) OR (cardiopulmonary
arrest) OR (ventricular fibrillation) OR (ventricular tachycardia)
Other terms:(Extracorporeal Membrane Oxygenation) OR (ECMO) OR (ECPR) OR
(Extracorporeal cardiopulmonary resuscitation) OR (ECLS) OR (Extracorporeal Life Support)
The search string used for the Cochrane search.
("heart arrest" OR "cardiac arrest" OR "myocardial infarction" OR "cardiopulmonary arrest" OR "ventricular fibrillation" OR "ventricular tachycardia"):ti,ab,kw AND ("ecmo" OR "extracorporeal membrane oxygenation" OR "ECPR" OR "extracorporeal cardiopulmonary resuscitation" OR "ECLS" OR "extracorporeal life support"):ti,ab,kw
The search string used for the EMBASE database.
exp "heart arrest"/ or exp "ventricular fibrillation"/ or exp "heart ventricle tachycardia"/ or ("ventricular fibrillation" or "ventricular tachycardia" or "heart arrest" or "cardiac arrest" or "myocardial infarction" or "cardiopulmonary arrest" or "ventricular tachycardia").ti,ab,kw. (449266), 2. exp "extracorporeal oxygenation"/ or ("extracorporeal membrane oxygenation" or "ECPR" or "extracorporeal cardiopulmonary resuscitation" or "ECLS" or "extracorporeal life support" or "ecmo").ti,ab,kw. (39803), 3. ("pigs" or "covid").ti,ab,kw. or ("shock" or "post-operative" or "surg*" or "transplant*" or "pediatric" or "paediatric" or "child*" or "neonat*" or "newborn" or "infan*" or "pregnan*" or "emboli*" or "hypotherm*").ti. (3278347), 4. (1 and 2) not 3 (4274), 5. 4 not (Conference Abstract or Conference Paper or Conference Review or Editorial or Letter).pt. (2392), 6. limit 5 to english (2265), 7. and PMID number already found in the other databases.
Acknowledgements
Not applicable
Availability of data and materials
Detailed documentation on the search strategy has been submitted as supplementary material.