
Asif Khan1, Ahmad Jaber2,3, Dima Siblini3,4, Aya Kawssan3,4, Nour Serhane3,4, Nasser Ismail3,4 , Ali Mokashar 3,4, Hiba Hamdar3*
1Shifa College of Medicine, Islamabad, Pakistan
2Gilbert and Rose-Marie Chagoury School of Medicine, Lebanese American University, Byblos, Lebanon
3Medical Learning Skills Academy, Beirut, Lebanon
4Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon
*Corresponding author: Hiba Hamdar, Medical Learning Skills Academy, Beirut, Lebanon.
Received date: December 07, 2023
Accepted date: January 16, 2024
Published date January 22, 2024
Citation: Asif Khan, Ahmad Jaber, Dima Siblini, Aya Kawssan, Nour Serhane et.al. (2024) “Empyema: idiopathic or delayed hypersensitivity due to latent tuberculosis: a rare case report and a review of the disease.” International J of Clinical Cardiology and Cardiovascular Interventions, 4(1); DOI: 10.61148/2836-2837/IJCCI/018
Copyright: © 2024 Hiba Hamdar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Empyema is a medical condition characterized by the accumulation of pus in the pleural cavity, which is frequently caused by infectious agents. It can have serious health consequences, with a variety of factors influencing its severity and prognosis. It could be caused by a bacterial infection or by chest trauma and other factors. Idiopathic empyema is uncommon. When a patient presents with empyema, medical professionals will typically look for underlying causes such as pneumonia, lung infections, or other relevant factors.
Case presentation
A 14-year-old male patient presented with symptoms of shortness of breath, orthopnea, fever, and cough, along with decreased breath sounds on the left side during physical examination. A chest X-ray revealed diffuse pleural effusion on the left side. A sample of pleural fluid was aspirated, showing high WBC count, low glucose, and elevated protein and LDH levels. Microscopic testing did not detect Mycobacterium Tuberculosis Bacteria or other suspicious microorganisms. The patient received broad-spectrum antibiotics for seven days and had 1300 ml of pleural fluid drained.
Conclusion
Empyema is not usually idiopathic, and its onset is frequently linked to other medical conditions or events, as mentioned in the search results. In our case, TB reactivation was most likely the primary cause.
bacterial infection ; chest trauma; idiopathic empyema; TB; teenager; adolescent health
Introduction
Empyema is a medical condition characterized by a buildup of pus in the pleural space (1). It can be primary empyema caused primarily by bacterial infection (2), a complication of untreated conditions such as parapneumonic effusion (3), or iatrogenic following thoracic surgery or trauma (4). We attempted to demonstrate in our case that empyema may be idiopathic because there was no clear pathology behind it; however, using medical critical thinking, we were able to demonstrate that empyema is not usually idiopathic, and its development is frequently linked to other medical conditions or events. The patient's age, underlying medical issues, and the timing of the presentation all influence how empyema manifests clinically (5). Nonetheless, the non-specific symptoms, which include cough, fever, dyspnea, and sputum production, may mimic those of pneumonia (6). The diagnosis of empyema typically necessitates a multimodal approach to confirm the condition, including imaging (such as chest X-rays and CT scans, which can reveal pleural effusion and help visualize the extent of empyema (7), laboratory testing, and thoracentesis, which involves withdrawing fluid from the pleural space in order to identify the causative agent and support the choice of appropriate treatment (8). Depending on the severity and stage of the condition, a combination of medical and surgical methods are used to treat empyema (9). We will demonstrate the tests that were performed, the pathogenesis of the condition, and the course of treatment in our case presentation.
Case Presentation
A 14-year-old male patient presented with one-week-long complaints of shortness of breath, orthopnea, fever, and cough. On physical examination, there was a noticeable decrease in breath sounds on the left side. Physical examination revealed no other noteworthy findings. The previously healthy young boy is up to date on vaccinations, has no notable medical history, and no history of surgical intervention or hospitalization was noted. In terms of family history, both his father and grandmother recently contracted Tuberculosis Mycobacterium Infection (TBI). On admission, blood was drawn for laboratory analysis, which revealed an elevated white blood cell (WBC) count of 26.3x10^9/L (granulocytes 83.9% and lymphocytes 11.9%) and platelet count of 419x10^9/L, respectively. After 3 days, the blood test was repeated for clinical assessment, and the WBC count and platelet count were dramatically reduced to 4.72x10^9/L (granulocytes 70.4% and lymphocytes 26.0%) and 214x10^9/L, respectively. A chest x-ray was also taken, which revealed diffuse pleural effusion on the left side. A 5 mL sample of pleural fluid was aspirated for testing. The fluid was visibly yellow, with a WBC count of 29000/cmm (neutrophils 98% and lymphocytes 2%), an RBC count of 50/cmm, a glucose level of 10 mg/dl, a protein level of 3.7 g/dl, and an LDH (lactate dehydrogenase) level of 12311 U/L. Furthermore, microscopic testing on the fluid revealed no Mycobacterium Tuberculosis Bacteria (MTB) or other suspicious microorganisms. After seven days of broad-spectrum antibiotic therapy, 1300 ml of pleural fluid was drained under local anesthesia. A 2 ml sample of aspirated pleural fluid was cytologically examined to rule out malignancy or chylothorax, and it revealed few mesothelial cells and no malignant cells. A second laboratory examination of a 20-ml pleural fluid specimen revealed a decrease in WBC count to 25000/cmm (neutrophils 90% and lymphocytes 10%) and an increase in protein level to 5.4 g/dl.
As a result of these findings, empyema was determined to be a certain diagnosis. However, no specific causative agent was identified.
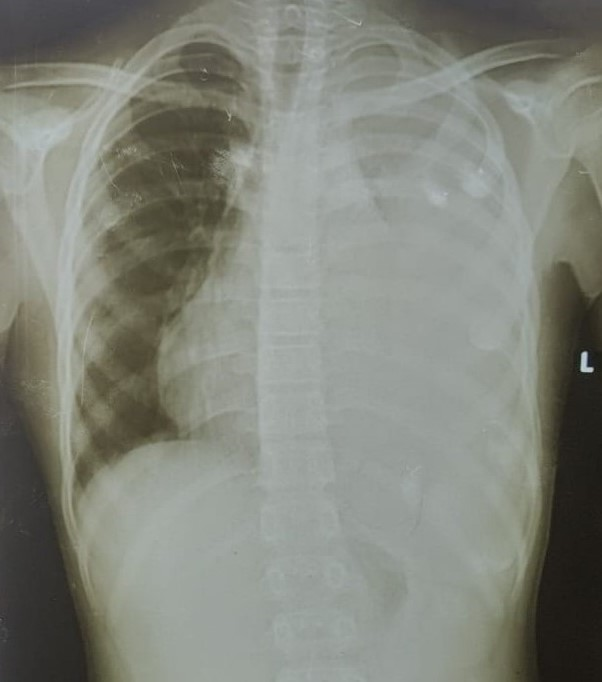
Figure 1: chest X-ray: the arrow indicates a thickened pleura and left pleural effusion.
Discussion
Although pediatric empyema is relatively uncommon, it is usually a complication of a secondary infection (10). The exact pathophysiology of the empyema was not fully understood, but it can be described in a series of hypotheses that we ranked from low to high evidence. The untreated pleural effusion and the patient having a prior infection that was not properly managed and has progressed to empyema and pus collection were the first hypotheses considered. It is well known that pleural effusion can progress to empyema when an infection, such as S.aureus, causes pus to accumulate in the pleural space (11). It takes at least 4 to 6 weeks for pleural effusion to progress to empyema (12). The effusion usually forms as a result of increased capillary permeability caused by endothelial injury, which results in neutrophil activation and membrane phospholipase products (13). If the outflow is not treated, it will accumulate in the pleural space, causing fibroblast accumulation and cytokines to progress to the fibrinopurulent stage, which will eventually evolve into empyema after 10 to 21 days (14). However, in our case, this is extremely unlikely, especially since the fluid culture revealed no infectious species. The second hypothesis we considered was the possibility of cancer. In some cases, empyema is caused by metastatic cancer (15). There are patients with cancer-related empyema (16). Although this is a rare occurrence, it is classified as a pleural cancer complication (16). As a result, cytological examinations should be performed. All laboratory tests, including erythrocyte/leukocyte counts and cultures, as well as an X-ray, indicated empyema in our case, and the cytological examination revealed no malignant cells, ruling out the possibility of malignancy. Other theories that were thought to be related to the pathogenicity were discussed, such as the existence of lung malformations, fistulas elsewhere, or other underlying genetic diseases like cystic fibrosis. However, all of these theories were ruled out because the patient displayed no other symptoms than respiratory ones, the X-ray revealed no lung malformations, and the patient has always been healthy with no overt symptoms or signs of cystic fibrosis.
However, the last hypothesis that we critically considered and analyzed was the reactivation of a dormant tuberculosis infection, especially given that two of his family members were infected with the disease. It may be placed into consideration that this patient at one stage was infected and got cured, specifically that the X-ray didn't show any signs, mainly as they are used to detect active TB but not latent one (17), additionally that neither specific blood test like QuantiFERON-TB or T-SPOT.TB tests, which usually detect the release of interferon-gamma in response to Mycobacterium tuberculosis antigen (18), nor the Mantoux Tuberculin Test (TST) was done. The question is whether reactivation of a latent TB infection can result in empyema. The answer to this question has been found in various studies, which confirm that the reactivation of latent TB can result in the development of TB empyema, a rare but well-documented complication of TB (19). However, a microscopic examination of the fluid revealed no bacterium. Can the patient develop empyema without demonstrating the etiological factor in the fluid? According to research by Vorster et al. (2015) (20), delayed hypersensitivity rather than a pleural space infection directly could be the cause of the pathogenesis (21). A paucibacillary mycobacterial infection that originates from initial parenchymal lesions and causes an immunological response that both increases pleural fluid formation and decreases pleural fluid removal is most likely the cause of the pleural effusion (22). The pleura first experiences a symptomatic, fast neutrophilic inflammatory response. The pleural effusion in this patient was with a high protein content, a neutrophilic predominance, and a high lactate dehydrogenase level. The pleura initially experiences a symptomatic rapid neutrophilic inflammatory response. This is followed by a prolonged lymphocyte-driven immune response, which includes the formation of pleural granulomas and the release of adenosine deaminase (ADA), which explains the increase in lymphocytes. As the effusion becomes lymphocyte-predominant and viable mycobacteria are contained, the likelihood of a positive pleural fluid culture decreases over time (20), which was the condition in our case.
Conclusion
Although TB empyema cases are uncommon in children, TB should be considered in the differential diagnosis of pleural effusion and empyema, particularly in patients with a family history of active TB infection. Empyema usually develops as a complication of another condition, such as pneumonia, lung infections, or pleural infections, and idiopathic empyema is uncommon. As a result, empyema is unlikely to be idiopathic. Additional research on the diagnosis and management of pleural complications in children and adolescents, such as TB effusions or empyema, is required.
Declarations