
Ghasem Zolfaghari
Department of Environment Science and Engineering, Faculty of Environmental Sciences, Hakim Sabzevari University, Sabzevar, Iran.
*Corresponding Author: Ghasem Zolfaghari, Department of Environment Science and Engineering, Faculty of Environmental Sciences, Hakim Sabzevari University, Sabzevar, Iran.
Received date: September 23, 2023
Accepted date: October 05, 2023
Published date: November 30, 2023
Citation: Ghasem Zolfaghari. (2023). “Removal of Chlorinated Phenolic Compounds from the Environment and Desulfurization of Oil with Carbon Nanoporous”. Environmental Pollution and Health, 1(1); DOI: 10.61148/EPH/001.
Copyright: © 2023 Ghasem Zolfaghari. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
In this research, SBA-16 nanoprocesses were first synthesized and then the surface of this nanostructure was modified using aminopropyltrimethoxysilane and melamine (Me-ASBA-16). The synthesis of SBA-16 has been carried out by two surfactants F127 and P123. In addition, Amino-ASBA-16 was used to remove 2,4-dichlorophenol from polluted water, and the effect of the initial concentration of the pollutant and contact time on the amount of pollutant absorption was investigated. Furthermore, Au-doped mesoporous carbon, Au-OCMK-3, was prepared for desulfurization and denitrogenation of refinery fuels. The synthesis of ordered mesoporous carbon, OMC, was performed using SBA-15 silica as the hard template and sucrose as the carbon source. The structural order and textural properties of the prepared materials were studied by X-ray fiffraction (XRD), Scanning Electron Microscopy (SEM), and N2 adsorption-desorption. Au-doped oxidized mesoporous carbon has been studied as a nanoabsorbent for removal of dibenzothiphene from n-heptane solutions as fuel model at room temperature. Batch adsorption studies were carried out to study the effect of contact time and initial concentration. The uptake capacity of dibenzothiphene and carbazole followed the order: Au- OCMK-3> CMK-3. The results showed that the synthesized compounds are suitable for removing phenolic and sulfur compounds.
amino-asba-16 ; desulfurization; 2,4-dichlorophenol; au-ocmk-3
1. Introduction:
Various studies have been conducted in the field of environmental pollution [1]. In recent years, the contamination of aromatic pollutants such as phenolic compounds in aquatic ecosystems has attracted increasing attention. Phenolic compounds are toxic and carcinogenic and cause unpleasant taste and smell in drinking water. The US Environmental Protection Agency has listed eleven types of phenols as highly toxic substances, among which chlorophenols are the most toxic and carcinogenic [2]. The reported sources of phenol pollution include rain water, surface water, underground water, soil, gases from burning wood, exhaust gas from cars, wastewater from many industries such as pharmaceutical, petrochemical, wood, metal forming, paint, electrical coating of surfaces, resins, plastics, rubber and glue industries [3-4]. Wastewaters containing phenolic compounds are considered a serious problem due to their high toxicity, low degradability and ecological aspects [1]. Contamination of soils and waters by fossil fuels or their derivatives is a widespread problem in many industrialized countries. Commercial gasoline and diesel contain a large amount of organic sulfur compounds with a concentration of 300 and 500 ppmw (parts per million by weight) [5]. One of the goals of the U.S. EPA is to decrease the content of sulfure in diesel oil to 30 ppm. These sulfur compounds present in the fuel oils can cause serious environment pollution because they will be turned into SOx species during combustion. Sulfur compounds also result in the severe corrosion of reactors and equipment in the oil processing step. It promotes investigation of new techniques of separations because low concentrations cannot be achieved using hydrodesulfurization (HDS). HDS is not able to remove aromatic hetrocyclic sulfur compounds to the required levels [6]. Thus, desulfurization, i.e. removal of organic sulfur compounds from fuel oils, is a required practice across the world and has become an important unit operation in petroleum refining [5]. Among those compounds, dibenzothiophene is considered as refactory species. Recently investigated methods have been oxidation with hydrogen peroxide in the presense of litanium oxides [7] or reaction-enhanced adsorption on undisclosed reactive adsorbent [8]. In the latter method, a proprietary adsorbent attracts sulfur and withdraws it from dibenzothiophene and an aromatic hydrocarbon is released to the system [9].
Some of the methods that have been used so far to remove the phenolic compounds from water sources include absorption, chemical oxidation, photolysis, sedimentation, filtering, osmosis, and ion exchange [10-11]. Recently, many types of nanostructured carbons have been produced through templating approaches [12, 13, 14]. Ryoo et al. reported the first preparation of new type of mesoscopically ordered carbon molecular sieves CMK-1 and CMK-3 (cubic and hexagonal, respectively) by carbonizing sucrose inside the pores of the cubic MCM-48 and hexagonal SBA-15 mesostructured silica materials [15, 16]. The ordered mesoporous carbon replicas of cage-like mesoporous silicas SBA-1 [17], SBA-7 [18], SBA-16 [19], and FDU-12 [20] were reported as well. Furthermore, chemical vapour deposition (CVD) was applied using liquid petroleum gas (LPG) as the carbon source and HMS as the template to prepare ordered mesoporous carbon (OMC) as well as carbon nanotubes (CNT) [21]. Also Anbeia and Moradi (2009) modified ordered mesoporous carbon with HNO3 and used for removing naphthalene-derived compounds [22]. Here, we report the preparation of CMK-3 from SBA-15 as hard template and sucrose as the carbon source. CMK-3 was modified with HNO3 and functionalized with gold solution by post-synthesis treatment. Au-doped oxidized mesoporous carbon, Au-OCMK-3, for removing dibenzothiphene from oil model was investigated. Furthermore inn this research, SBA-16 nanostructure was first synthesized and then the surface of this nanostructure was modified using 3-aminopropyl trimethoxy silane (ASBA-16). Next, the resulting material was treated with melamine resins (Me-ASBA-16). The synthesized material was used to remove 2,4-dichlorophenol from polluted water.
2. Exprimental:
2.1. Materials:
The reactants used in this study were gold solution (HAuCl4), tetraethyl orthosilicate (TEOS) as a silica source, Pluronic® P123 (EO20PO70EO20) and F127 (EO106PO70EO106) as surfactant, H3PO4 (85%), deionized water for synthesis of mesoporous silica (SBA-15), sucrose as a carbon source, and sulfuric acid as a catalyst for synthesis of ordered mesoporous carbon.
2.2. Synthesis of SBA-15, SBA-16, and modification:
The method of Kim et al. [15] was used for the synthesis of SBA-16. The synthesis procedure of SBA-15 involves the use of Pluronic P123 (EO20PO70EO20; BASF) as the template agent, which is dissolved in distilled water and H3PO4 (85%) (Aldrich) obtaining initial solutions with the following molar compositions: 1:0.017:1.5:208 SiO2/P123/H3PO4/H2O [24]. To modify SBA-16 with propylamine, 2 g of SBA-16 is spread in 70 mL of dry toluene, and then 1 g of aminopropyltrimethoxysilane is added to the mixture under nitrogen vacuum (Amino-SBA-16) [25]. Diisopropylethylamine was used for the for the synthesis of melamine resins on silicate nanoprous modified with propylamine (Me-SBA-16).
2.3. Synthesis of CMK-3 and functionalization:
The synthesis method for CMK-3 was to dissolve 1.25 g sucrose and 0.14 g H2SO4 in 5.0 g H2O, and to add this solution with 1 g SBA-15 [15]. The texture and surface chemistry of synthesized CMK-3 was modified by means of oxidation treatment in liquid phase. Au-doped OCMK-3 was prepared by mixing 5000 ppm of HAuCl4 and OCMK-3 for 10 h. The solid was separated by filtration, washed and vacuum dried. These samples were reduced to form nanoparticles of Au using 0.1 M NaBH4 solution.
2.4. Adsorption tests:
To investigate the adsorption process, the amount of 0.9 g/L of the synthesized adsorbent was mixed with solutions of 2,4-dichlorophenol in concentrations of 10, 20, 30, 50, 100, 150 and 200 mg/L and in time 15, 30, 60, 120, 180, 240 and 300 minutes were mixed by a mechanical stirrer. Then the adsorbent was separated from the solution with the help of filter paper and the amount of absorption was measured (adsorbent dose = 0.9 g/L, temperature: 40 ºC, and pH = 7). Also, to investigate the effect of adsorbent dose, the amounts of 0.2, 0.4, 0.6 and 0.8 g/L were tested at a concentration of 10 mg/L of the pollutant at a temperature of 40 ºC and a pH of 7. Pollutant adsorption percentage (R) was calculated using following equation:
R = C0-CeC0×100
where C0 and Ce are the initial and equilibrium concentrations of the compound in mg/L, V is the solution volume in L, and m is the adsorbent mass in g. Measurement of absorbance was analyzed by UV-Vis spectrophotometer. In the case of Au-OCMK-3, the absorption process was carried out under the same conditions.
3. Results and discussion:
Figure 1 shows the XRD pattern obtained for SBA-16, Amino-SBA-16 and Me-SBA-16 compounds. These three materials show regular silicate structure. In this figure, three XRD peaks corresponding to the reflections of 110, 200 and 211 of the three-dimensional space group are observed. The matching of the XRD pattern found in the reported studies for the compound SBA-16 proves the correctness of the synthesis of this compound. The low-angle XRD patterns of CMK-3 and Au-OCMK-3 are shown in Fig. 1. The nitrogen adsorption–desorption isotherms performed at 77K for the CMK-3 before and after modification are showed in Fig. 2. Both mesoporous materials yield a type IV isotherm. The type IV isotherm (IUPAC classification) is typical for mesoporous systems [26].
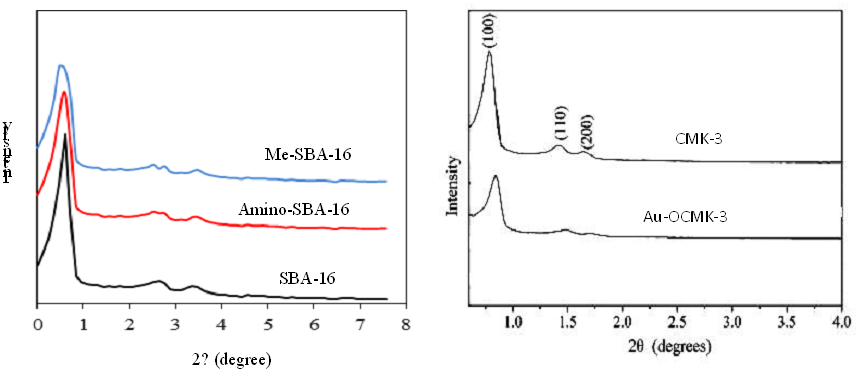
Figure 1: XRD patterns of synthesized materials.
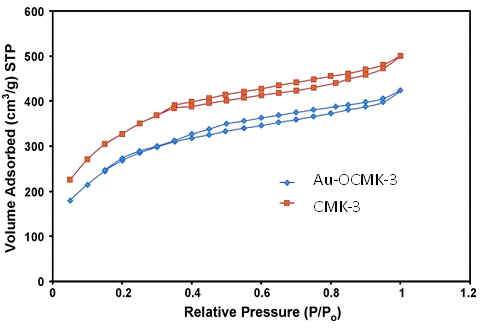
Figure 2: Nitrogen adsorption–desorption isotherms of CMK-3 and Au-OCMK-3.
In order to determine the equilibrium time in the absorption of 2,4-dichlorophenol, the amount of absorption has been studied as a function of time and in different initial concentrations (Fig. 3A). After 2 hours, increasing the contact time has no effect on increasing the amount of absorption, and this indicates that the reaction has reached equilibrium. It can be seen that the absorption process is fast at first and becomes slower as the equilibrium time is reached [27]. This is related to the fact that in the initial stages of adsorption, more active sites are available, so the adsorption happens quickly, after this time, due to the repulsion between the adsorbed species and the species in the mass, the adsorption becomes difficult. Adsorption in different concentrations has also been shown. The amount of adsorption decreased with increasing concentration. To investigate the effect of adsorbent amount on absorption capacity, different amounts of Me-SBA-16 adsorbent (0.2, 0.4, 0.6 and 0.8 g/L) were added to 25 mL of 200 mg/L solution of the pollutant. Figure 3B shows that the amount of absorbed phenolic compound increases significantly with increasing amount of absorbent.
The desulfurization performance of as-received and modified mesoporous carbon was valuated using model oils in a batch type adsorption setup. For a typical run, the adsorption conditions are as followes: room temperature; adsorption time 30, 60, 120, 180 and 240 min. The sorption of dibenzothiphene on Au-OCMK-3 was more than CMK-3 (Fig. 4). Au species contribute to an increase in the number of active centers, which improve the adsorption of dibenzothiphene on mesoporous carbon. The results of the study conducted by Zolfaghari et al. [28] show that OCMK-3 functionalized with zinc oxide is an effective adsorbent for removal of lead and mercury.
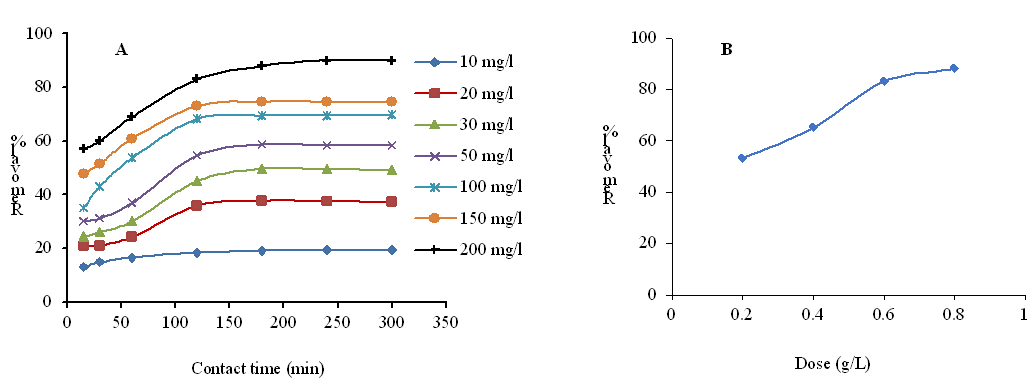
Figure 3: The effect of initial concentration and time on the absorption of 2,4-dichlorophenol by Me-SBA-16 (adsorbent dosage = 0.9 g/L, temperature: 40 ºC, pH = 7) (A) and the effect of adsorbent dose on the absorption of 2,4-dichlorophenol by Me-SBA-16 (initial concentration= 10 mg/L, contact time: 120 minutes, temperature: 40 ºC, pH = 7) (B).
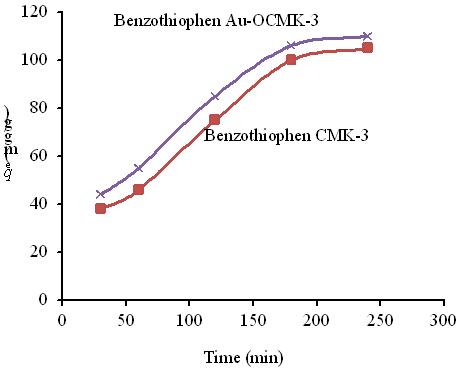
Figure 4: The sorption of dibenzothiphene on nanoadsorbent studied (Effect of contact time on removal of DBT (dose of 0.7 g/L and temperature of 25 ºC).
4. Conclusion:
In this research, the synthesis of nanoporous silicate SBA-16 has been done correctly. The XRD diagram confirms this issue. Me-SBA-16 showed that it has a high ability to absorb 2,4-dichlorophenol. Melamine groups are the reason for this ability. Further researches on this matter of identification and investigation of different dimensions of adsorption are ongoing. We demonstrate that functionalization of CMK-3 with gold is possible. The present study shows that the Au-OCMK-3 some deal is an effective adsorbent for the removal of dibenzothiphene.
Acknowledgement:
We sincerely thank Iran Nanotechnology Initiative Council. We also appreciate National Elite Foundation of Iran.