
Njoku-Obi *, Treasure
Department of Microbioogy, Faculty of Biological Science, Imo State University, Owerri.
*Corresponding authors: Njoku-Obi, Department of Microbioogy, Faculty of Biological Science, Imo State University, Owerri.
Received Date: June 06, 2024
Accepted Date: June 15, 2024
Published Date: June 17, 2024
Citation: Njoku-Obi, Treasure, (2024). “Bacteriological Analysis of Wastewater Collected from Specific Hostels at Imo State University, Owerri”. Clinical Research and Clinical Case Reports, 5(2); DOI: 10.61148/2836-2667/CRCCR/78.
Copyright: © 2024 Njoku-Obi, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The purpose of this study was to examine the bacterial contamination of wastewater that was collected from particular Imo State University dorms in Owerri. Water samples were taken from ten (10) distinct hostels at Imo State University in Owerri, Imo State. Each wastewater sample was serially diluted ten times in sterile physiological saline up to a dilution of 10^6. An aliquot of 0.1 ml from each dilution (10-2 and 10-4) was then inoculated on newly prepared nutrient agar plates, MacConkey broth, Salmonella shigella agar, and thiosulpate citrate bile salt agar. For a duration of 24 hours, the cultivated plates were incubated at 35°C to isolate bacteria. For accurate isolate identification, colonies were put through biochemical assays, identification using live gram staining, and sub-culturing. Result from heterotrophic count showed bacteria distribution and percentage occurrences in wastewater from restaurants indicating that Staphylococcus spp showed higher occurrence 18(31.5%), followed by Klbsiella spp 12(21.1%), Escherichia coli 11(19.3%). Salmonella sp 9(15.8%). Vibro spp 7(12.3%) which showed the least number of occurrence while fungi isolates of Penicillium sp showed higher occurrence 6(60%), and Aspergillus sp 4(40%), which showed the least number of occurrence. The study therefore concludes that microorganisms are present in wastewater from hostels and it is recommended that sources of disposal of wastes I.e water and dirts from dishes should be located far away from the environment to avoid fecal contamination. This will lead to a specific goal of defending the health of individuals within the localities.
bacteriology; analysis; wastewater; hostels
Introduction
Water is a universal resource that is frequently misused and taken for granted due to its free availability in nature, particularly in third-world countries where knowledge is not easily available or shared with the public[1].Despite its seeming abundance, pure water is one of the rarest elements on Earth. Similar to other limited resources, water is governed by laws, policies, and regulations to avoid misuse and to govern its ownership, exploitation, preservation, and sustenance [2]. The necessary water quality criterion is determined by the intended use of the water [3].
For obvious reasons, water used for fish farming would not meet the same standards as water intended for human consumption, food, or pharmaceutical industry use.
Any water whose quality has been negatively impacted by human activity is considered wastewater. It is made up of liquid waste that is released from commercial, industrial, and/or agricultural facilities as well as from residential buildings. The toxins and concentrations in it can vary greatly [4]. Wastewater is utilized water that has been contaminated by things like chemicals, oil, soap, food scraps, and human waste. This includes water from toilets, washing machines, dishwashers, showers, and sinks in households. Industries and businesses also contribute a portion of the used water that needs to be cleaned. Storm runoff is also included in wastewater.
The rain that falls on the street during a storm is not clean, despite what some people believe.Our rivers and lakes may be harmed by hazardous material that runs off of parking lots, roadways, and rooftops [5].Microorganisms are ubiquitous, which facilitates their growth in a variety of environments, including soil that contains waste water.The daily increase in waste water from homes, businesses, and even farms is a result of population growth [6]. Municipal sewage treatment plant effluent and sludge, as well as plant-derived water, are known to contain high concentrations of heavy metals and micro- and macronutrients. These contaminants can contaminate soil, endangering beneficial microorganisms for agriculture.
This surface pollution is caused by agricultural operations, spills, the discharge of both liquid and solid waste, and the percolation of surface pollutants via unsaturated
The majority of nutrients in wastewaters are both organic and inorganic. Among the inorganic concentrations are potassium, phosphate, nitrogen, and other elements. Consequently, harmful or deadly heavy metals, the content of which varies depending on the environment, are present in waste waters in relatively high concentrations [8].
Water was used so extensively that waste water was defined differently in every country and continent. The prior study characterized waste water as a mixture of one or more domestic effluents, including gray water from cooking and bathing waste and black water from excreta, urine, and fecal sludge.
It can also refer to the water from businesses and organizations such as hospitals, as well as storm and urban runoff and industrial effluent. Waste water is also defined as the dissolved or suspended particles from agricultural, horticultural, and aquaculture effluent [9]. Furthermore, water that has been tainted by a biological or chemical contaminant is frequently unsuitable for drinking and other use [10]. The principal source of waste disposal, agricultural fields and river systems, are clearly receiving improperly managed waste water. The physical, chemical, and biological characteristics of the soil that water flows through both before and after it enters rivers can be changed, particularly by industrial effluents and their surroundings [11].
Moreover, the main causes of pollution in all settings are the industries. Even though different industries can release different amounts of pollutants into the environment through different channels, wastewater from those industries includes the sanitary waste of their workers, manufacturing process wastes, wash waters, and relatively clean water from heating and cooling operations [12].
River water systems with high pollution levels are unfit for aquatic life, drinking, irrigation, and other uses because they increase total dissolved solids (TDS), total suspended solids (TSS), biological oxygen demand (BOD), chemical oxygen demand (COD), and fecal coli formation [13].
Consequently, biodegradable wastes from tanneries, pulp and paper industries, textiles, slaughterhouses, human sewage, plating shops, and hair salons contribute to the high biochemical oxygen demand (BOD) of industrial wastewaters [14]. Despite the diversity of its constituents—which may be the result of organic contamination of inland water systems in continents like Africa—soil has shown to be a favorable environment for bacteria. These soil bacteria are especially vulnerable to environmental problems in Africa due to high poverty, economic underdevelopment, and social underdevelopment [15].
But, because of the dense populations living there, the issue of waste water disposal has emerged as one of the major problems facing urban areas. Due to this, sewage-related issues are now a global concern [16]. According to Nkansah, Onwusah, and other researchers, contaminated soil water reduces oxygen's availability as an electron acceptor, which in turn causes a decrease in the amount of accessible nitrate that turns into gaseous nitrogen and has negative environmental repercussions. Additionally, pollutants in waste water can seep into the soil and change its chemical makeup, leading to air and land pollution as well as a number of negative effects on the environment and human health from inadequate waste water treatment [17].
Most research on waste-water-contaminated soil examined the interactions between the local microbial community and the relevant environmental parameters. A few studies, however, compared the microbial community in a polluted environment to that of an environment under investigation where the microbial communities were located at varying distances from the pollutant [18, 19]. A new danger to agriculture and microbiology is the contamination of soil in farmed fields by toxic heavy metal-laden industrial effluents. Thus, the purpose of this research.
Materials and methods
Study area
Owerri is the capital of Imo state in Nigeria, set in the heart of Igboland. It has an estimated population of about 750,000 as of 2016 and is approximately 100 square kilometers (40 sq mi) in area. Owerri is bordered by the Otamiri River to the east and the Nworie River to the south . This study was carried out in Hostel areas around Imo State University is a State University situated in Owerri, Imo state, Nigeria.
Materials
The materials used for this study were: waste water samples, autoclave, Petri-dishes, cotton wool, Nutrient agar, MacConkey agar, Salmonella Shigella agar, inoculating loop, distilled water, slant bottles, beakers, measuring cylinders, jars, normal saline, pipette, tube rack, masking tape, gram staining kit, measuring scale, aluminum foil, spatula, spirit lamps and test tubes.
Sample Collection
Wastewater samples were collected in duplicate from five different hostels for the study within Imsu Front gate Hostel alliance and the samples were labeled ‘A’ to ‘E’. The waste water samples were collected into 250ml sterile screw-cap containers and transported to the Microbiology Laboratory of the Imo State University, Owerri for analysis within 2-3 h of collection.
Microbiological Analysis
Sterilization Technique
The materials used for this study were sterilized using standard techniques. Glass wares were sterilized in the hot air oven at 160oC for 1 hour. Culture media except SSA were sterilized by autoclaving at 121oC 15psi pressure unit for 15 minutes. Inoculation wire loop was sterilized by flaming intermittently to red hot over a Bunsen flame. Glass rod spread (hockey stick) was sterilized intermittently by dipping in absolute alcohol and bringing it over a burning flame to burn off. Bench top, inoculation hood and working area was sterilized by disinfecting with purit antiseptic and covering with 75% ethanol. Sterile disposable hand gloves and face masks were worn and changed after each procedure to ensure aseptic conditions.
Preparation of Media
The media used for this study was prepared according to the manufacturers’ instructions.
Serial Dilution and Inoculation of Sample
An aliquot (1ml) of the wastewater was transferred into 9ml of distilled water, thoroughly shaken using a vortex and serially diluted up to 10-4 dilution. 1ml of dilutions 10-2 and 10-3 were inoculated in duplicates onto Nutrient agar, MacConkey agar and Salmonella Shigella agar plates respectively.
Total Heterotrophic Bacteria Count (THBC)
To enumerate the total heterotrophic count, the samples were serially diluted and the dilutions were inoculated on a Nutrient Media. The inoculated medium was incubated for 24hours at 37oC. After the period of incubation the colonies were counted and recorded at colony forming unit per mil of sample (cfu/ml).
Total Coliform Count (TCC)
MacConkey Agar was used in the enumeration of coliforms. This was achieved by the inoculation of the diluted sample on this medium. The inoculated medium was incubated and number of colonies were counted and recorded as cfu/ml after the period of incubation which was done for 24hours at 37ᵒC.
Total Salmonella-Shigella count
0.1ml aliquots of the serially diluted samples was plated unto Salmonella Shigella agar (SSA) for enumeration of total Salmonella Shigella count. The SSA plates were incubated at 370C for 24hours. After the incubation period, colonies which developed on the plates were counted and recorded as colony forming units per milliliter (CFU/ml) of the sample.
Sub-Culture
After successful growth of microorganisms the pure cultures of bacteria were streaked on freshly prepared nutrient agar and incubated at 37oC for 24 hours to achieve vigorous growths of bacteria.
Identification Of Bacteria Isolates
The colors and mode of development of the isolates were noted. The isolates were further subjected to Gram staining in order to determine their reactions to Gram's reagents and other biochemical tests. The Gram stained bacterial isolates were prepared and identified using colonial and cellular characteristics, then biochemical properties.
Gram Staining
A thin smear of the bacteria culture was made, air-dried and heat-fixed by passing the underside of each slide over Bunsen burner flame for about four times. The slides were placed on a staining rack fixed smear were covered with crystal violet stain for one minutes. The stains were washed off with clean distilled water. The water was drained off and the smear was flooded with Gram's iodine and allowed to stand for one minute. The iodine was washed off with clean distilled water and the complex was decolourized with ethanol for 30 seconds and quickly washed with clean distilled water. The smear was covered with safranin for 30 second. The stain was washed off with clean distilled water and blotted dry. It was examined first with X40 objective to see the distribution of the organisms and then oil immersion objection to observe the bacteria.
Biochemical Tests
Biochemical tests are tests used for the identification of bacteria species based on the differences in the biochemical activities of different bacteria. Biochemical techniques will be used to test the bacteria isolate for its ability to ferment certain sugar, produce certain enzymes like oxide, catalase, indole, methyl red, and utilize citrate as their carbon source, Gram staining and motility as means of their identification. Biochemical tests will be performed according to standard procedure of Cappuccino and Sherman.
A. Motility Test
This test was employed to determine motility of bacteria. Most bacteria move by flagella, which is the outer appendages of plasma membrane and cell wall. Motility test agar was stabbed with inoculum in a sterile condition and incubated at 37°C for 48 hrs. Growth was observed after completion of incubation period, a diffused growth spreading from line of inoculation or diffusion at some points along the stab line was considered motility and growth along the line without any diffusion was considered non – motile.
B. Indole Test
This test was used to check ability of the organisms to form indole from tryptophan or to detect the presence of enzyme tryptophanase which converts tryptophan to indole. One percent tryptophan broth in a test tube was inoculated with bacteria colony. After incubation period of 37°C for 48hours, then one militre (1ml) of chloroform was added to the broth. The test tube was shaken gently, then 2.1 of Kovac’s reagent was added and this was also shaken gently and allowed to stand for twenty (20) minutes. The formation of red coloration at the top layer indicated positive and yellow coloration indicated negative.
C. Oxidase Test
This was carried out to identify bacterial species that produced the cytochrome oxidase enzyme. Pseudomonas aeruginosa and Escherichia coli were employed as positive and negative controls respectively.
To detect presence of the enzyme, oxidase in the bacteria was performed. It catalysed transport of electrons between bacteria and the redox dye (methylene blue). Few drops of methylene blue was added to 72 hour culture in nutrient broth media. Positive reaction was indicated by change in colour of the broth to colourless within few seconds.
D. Methyl Red Test
The test was used to detect acid production from glucose. Production of acid lowers the pH of the medium below 4.2 which was detected by the pH indicator methyl red. Bacteria was inoculated into tubes containing methyl red- voges proskauer (MRVP) broth and incubated at 30 ±0.1°C for 72 hours. Some small quantity (2-3 drops) of methyl red test was added. A red colour indicated positive reactions.
E. Vogues - Proskauer Test
Ability of many bacteria to ferment carbohydrates (especially glucose) with production of acetyl methyl carbinol reduction product into neutral products and carbon dioxide instead of organic acid is assessed. Bacteria was inoculated into tubes containing MRVP broth and incubated at 30±0.1°C for 72 hours. After incubation a mixed solution α-napthol and potassium hydroxide was added to 2.5 to 5 ml of culture. Development of crimson red colour of the medium indicated positive result.
F. Citrate Utilization Test
Ability of the bacteria to grow in a medium containing citrate as sole source of carbon and energy source is detected. Citrate utilization was monitored by appearance of growth and increase of pH from 6.8 which was indicated by the change in color of bromothymol blue indicator of the medium. This was carried out by inoculating the test organism in test tube containing Simon’s citrate medium and this was inoculated for 24 hours to 72 hours. The development of deep blue color after incubation indicated a positive result. No growth and yellowish-green color of the slant indicated negative result.
G. Catalase Test
Presence of enzyme catalase which catalyzes breakdown of hydrogen peroxide into water and oxygen was studied on culture plates of nutrient agar flooded with hydrogen peroxide solution. This was carried out by putting a drop of hydrogen peroxide on a clean slide. With the edge of another slide, a colony of the organism was picked and allowed to be in contact with the hydrogen peroxide. Presence of bubbles indicated positive reaction while absence of bubble indicated negative reaction.
H. Sugar Fermentation Test
This test was used to determine the ability of bacteria to utilize different sugars. Examples are mannitol, glucose, lactose and sucrose.
Materials – Sugar solution, test tube, Durham tube
The four sugar solutions were prepared and poured into test tubes well stopped with Durham tube for gas collection. The sugar is autoclaved after which a loopful of test organisms is introduced into the sugar solution. A change in color from pink to yellow shows fermentation and collection of gas bubbles in the Durham tube shows gas production which is a positive test. A control was set up without the organism inoculated.
Results
The wastewater samples collected randomly from five different hostels within Imsu Front gate Hostel alliance were carefully analyzed for their microbial profile.
The mean total viable count (TVC) ranged from 3.9 x 104 – 6.9 x 104 cfu/ml, mean total coliform count (TCC) ranged from 2.8 x 104 – 6.0 x 104 cfu/ml and the mean total Salmonella Shigella count (TSSC) ranged from 1.7 x 104 – 3.8 x 104 cfu/ml as shown in Table 1.
Table 1: Mean Total Aerobic Microbial Population in the Wastewater Samples (cfu/ml)
|
Sample |
Total Viable Count (TVC) |
Total Coliform Count (TCC) |
Total Salmonella Shigella Count (TSSC) |
|
A |
6.9 x 104 |
6.0 x 104 |
3.1 x 104 |
|
B |
5.2 x 104 |
4.1 x 104 |
2.5 x 104 |
|
C |
5.8 x 104 |
5.3 x 104 |
3.8 x 104 |
|
D |
4.2 x 104 |
2.8 x 104 |
1.7 x 104 |
|
E |
3.9 x 104 |
3.0 x 104 |
NG |
Key points:
NG – No growth
Cfu/ml – colony forming unit per ml
Table 2 shows the Morphological and Biochemical Characteristics of the bacterial isolates from the Hostel waste-water Samples. The suspected bacteria isolates identified from the waste-water samples were: Staphylococcus sp, Pseudomonas sp, Escherichia coli sp, Salmonella sp and Shigella sp.
Table 2: Morphological and Biochemical Characteristics of the Bacterial Isolates from the Waste-water Samples
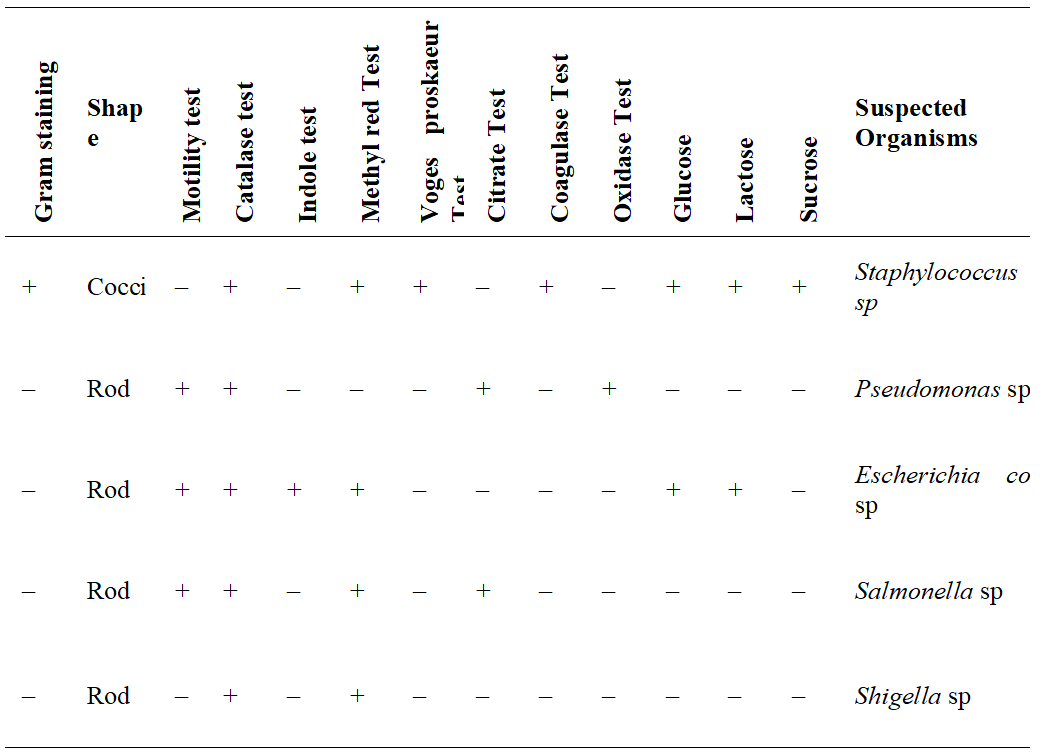
Keys: - Negative, + Positive
The result shows that Staphylococcus sp (30.3%) was the most prevalent bacteria organism having an occurrence in all ten (10) waste-water samples used for the study. The Pseudomonas sp (24.2%) was the second most isolated bacteria organism having an occurrence in eight (8) waste-water samples followed by Escherichia coli sp (21.2%) and Salmonella sp (15.2%) which were isolated from seven (7) and five (5) samples respectively. The least isolated organism was the Shigella sp (9.1%) which had an occurrence in three (3) samples respectively. The frequency and the incidence of these isolated bacteria organisms are given in Table 3 below.
Table 3: The Frequency and the Incidence of the Bacterial Organisms Isolated from the Waste-Water Samples
|
SN |
Bacteria Isolate |
Frequency (Occurrence In Samples) |
Percentage % |
|
1 |
Staphylococcus sp |
10 |
30.3 |
|
2 |
Pseudomonas sp |
8 |
24.2 |
|
3 |
Escherichia coli sp |
7 |
21.2 |
|
4 |
Salmonella sp |
5 |
15.2 |
|
5 |
Shigella sp |
3 |
9.1 |
|
|
Total |
33 |
100 |
Percentage = No of OccurrenceTotal no of occurrence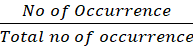 x 100
x 100
Discussion
Five distinct bacterial species were recovered for this investigation from waste water samples collected from dorms near Imo State University's Front Gate Alliance in Owerri. The physical characteristics and biochemical analyses carried out for their identification provided information about the organisms. They consist of Salmonella sp., Shigella sp., Escherichia coli sp., Pseudomonas sp., and Staphylococcus sp. The physical and biochemical characteristics examined to identify the isolates of bacteria are displayed in Table 2.
The data presented in Table 1 indicates that samples A had higher total viable count and coliform count, respectively. Similarly, mean total coliform count (TCC) ranged from 2.8 x 104 – 6.0 x 104 cfu/ml and mean total Salmonella Shigella count (TSSC) ranged from 1.7 x 104 – 3.8 x 104 cfu/ml.The unhygienic conditions of the surroundings may be the cause of the elevated microbiological counts seen in this dormitory.The low quantity of bacteria found in sample E's wastewater samples when compared to other sample regions is noteworthy. This could be related to the environment's general degree of hygiene as well as attempts at various sanitation exercises.
As shown in the study of [20], Staphylococcus aureus was isolated from every sample of waste water. Among the significant bacteria that have been linked to human illnesses like foliculitis, carbuncles, scalded skin syndrome, and boils is Staphylococcus aureus. Extensive microbiological diversity, including species of Staphylococcus aureus on human hair, has been described and suggested in literature [21]. Staphylococcus aureus is a common human flora and has the ability to spread infection [22].
Since pseudomonas is a highly adaptive bacterium that can be found in a wide range of conditions, including distilled water, the discovery that it was recovered from eight out of the 10 samples was not concerning [23]. Additionally, it has been observed in individuals with compromised or malfunctioning immune systems, suggesting that these microorganisms may have originated from pupils experiencing immune system impairment [24].
Escherichia coli sp. is an indicator organism of faecal contamination and a member of the Enterobacteriaceae family. It was isolated from several hostel locations.
Additionally, it is noted that the disposal of hostel wastewater poses a potential risk to the environment in terms of ugliness, contamination of the land and water, and decreased air quality due to the release of unpleasant odors and various gases from anaerobic decomposition as well as sporadic burning. Because it encourages the dispersion of bacterial infections into the air, either as free entities or attached to particles, it also poses a risk for air pollution and contamination.
While these pathogens are not as serious when suspended in the air, they cause a variety of infectious diseases, respiratory symptoms, and lung function impairment when they settle on surfaces. These conditions can range from mild, acute conditions that barely affect daily life to severe, chronic respiratory diseases, cancer, etc. that require specialized care. International rules state that water with this degree of contamination is not fit for household use and should not be released untreated into the environment [25].Household wastewater is dumped into untreated open drainages, and the leachates from these wastes' gradual breakdown can introduce enteric bacteria into the river, which can lead to gastrointestinal diseases.
The wastewater's high organic matter content promotes the quick growth of oxygen-consuming microbes, which causes the water to lose its dissolved oxygen and become septic or anoxic, which is fatal to aquatic life [26].
Conclusion
Both individuals and the government should discourage the careless release of untreated waste water into the environment from these residential sources. To ensure that the wastewater from these domestic sources is appropriately treated and disposed of, mechanisms for doing so should be devised. Pathogen isolation from wastewater is a sign that the hygiene regulations in place are insufficient. This requires public health organizations to raise awareness and provide for seminars, training sessions, and talks for the operators, employees, and residents of these towns.