
Maria L. Nord1*, Mark D. Mintline2, Victor P. Celis3, Shawn A. McClure4
1Private Practice Oral and Maxillofacial Surgery Previous Nova Southeastern University 17121 Marcy Street,Suite 102 ,Omaha, NE 68118 USA.
2College of Dental Medicine Coordinator of Advanced Oral Diagnosis Workgroup, Assistant Professor, Pomona, CA 91766-1854.
3Private Practice Oral and Maxillofacial Surgery Previous Nova Southeastern University,3200 South University Drive, Fort Lauderdale, FL 33328-2018.
4Residency Program Director Associate Professor College of Dental Medicine at Nova Southeastern University Department of Oral and Maxillofacial Surgery Health Professions Division ,3200 South University Drive, Fort Lauderdale, FL 33328-2018.
*Corresponding authors: Maria L. Nord, Department Private Practice Oral and Maxillofacial Surgery Previous Nova Southeastern University,17121 Marcy Street,Suite 102 ,Omaha, NE 68118 USA .
Received Date: March 02, 2024
Accepted Date: March 05, 2024
Published Date: April 10, 2024
Citation: Maria L. Nord, Mark D. Mintline, Victor P. Celis, Shawn A. McClure, (2024). “Angiomatoid Fibrous Histiocytoma of the Mandible: A case report and review of literature”. Clinical Research and Clinical Case Reports, 5(1); DOI: http;//doi.org/08.2024/1.1077.
Copyright: © 2024 Maria L. Nord, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background:
The purpose of this article is to review the current literature on angiomatoid fibrous histiocytoma (AFH) and present a case study in which it presented intraosseous in the mandible of a young, female patient. Reported ages ranged from 6 months to 83 years. The size of the masses ranged from 0.5 cm to 10 cm. In our research, 49 were male, 41 female and one individual’s sex was not specified. AFH is a relatively rare sarcoma that typically affects the somatic tissues of extremities. We present an additional case report showing a rare intraosseous AFH of the mandible in a young female patient. The lesion was treated successfully by segmental mandibular resection and perhaps this case can aid in both the diagnosis and treatment of these lesions with bony invasion of the jaws.
Conclusion:
Shi et. al noted most AFH masses to be painless and slow growing. [9] Non-specific symptoms such as fever, malaise, anemia, weight loss may also be present. [4, 5] AFH has four distinct histopathological characteristics: 1) spindle to ovoid shaped histiocytoid tumor cells, 2) central cystic space often filled with blood, 3) a lymphoplasmacytic rim, and 4) a dense fibrous pseudocapsule. [3, 6] Most AFHs are diagnosed based on histopathology. When in doubt, immunohistochemistry, and molecular studies aid in the diagnosis. The tumor cells are positive for: CD68, epithelial membrane antigen, desmin, and CD99. [7, 8] and molecular studies show that over 90% of cases exhibit a t(2;22)(q33;q12) translocation resulting in an EWSR1-CREB1 fusion. Less common genetic abnormalities include the t(12;22)(q112;q12) translocation resulting in an EWSR1-ATF fusion and cases that harbor a FUS-ATF1 fusion. [9, 11] AFH is exceedingly rare in the maxillofacial region and is even more so when found in the jaws. [7,8] Since there are over 50 classified subtypes of sarcoma and an inability to adequately perform clinical trials on each rare subtype, case reports such as this are important in the understanding of tumor behavior, characteristics, genetics, treatment modalities and prognosis. [4]
angiomatoid fibrous histiocytoma; fibrous histocytoma; AFH; intraosseous sarcoma; head and neck sarcoma; soft tissue sarcoma
Introduction:
The purpose of this article is to review the current literature on angiomatoid fibrous histiocytoma (AFH) and present a case study in which it presented intraosseous in the mandible of a young, female patient. A review of literature was completed using the Medline, EMbase, and Google Scholar databases using the keywords: angiomatoid fibrous histiocytoma, fibrous histiocytoma, AFH, head and neck sarcoma, benign fibrous histiocytoma, malignant fibrous histiocytoma. Very few articles exist that reference angiomatoid fibrous histiocytoma (AFH). Relevant articles were selected and included in the review. Fifteen articles were included. Reported ages ranged from 6 months to 83 years. The size of the masses ranged from 0.5 cm to 10 cm. In our research, 49 were male, 41 female and one individual’s sex was not specified. AFH is a relatively rare sarcoma that typically affects the somatic tissues of extremities. It is relatively rare in the head and neck, and when diagnosed in this region, little literature exists which discusses behavior of the tumor, treatment modalities, complications, and recurrence rates. We believe an additional case report showing a rare intraosseous AFH of the mandible in a young female patient. The lesion was treated successfully by segmental mandibular resection and perhaps this case can aid in both the diagnosis and treatment of these lesions with bony invasion of the jaws.
Case Report:
A 9-year-old African American female with no relevant medical history presented with a mild tenderness of the right mandibular angle that reportedly started two days prior to presentation. On examination, the patient had a firm, non-erythematous, mass localized to the right mandibular angle (Figure 1). No history of trauma to the mandible was reported and the patient did not report paresthesia. A thorough exam and review of records showed she was up to date on her vaccinations. Growth and developmental milestones were met appropriately. On a computerized tomographic (CT) scan neck soft tissue with IV contrast, a 1.9 x 2.4 x 1.9 cm mixed cystic and solid lesion was identified at the level of the right mandibular angle arising likely from the bone with an associated periosteal reaction. The patient was admitted for biopsy. She was taken to the operating room where an attempt at an incisional biopsy was made from an intra-oral approach. The surgeons encountered approximately 50 ccs of blood and decided to convert to an extra-oral, submandibular approach for appropriate access and visibility. A soft tissue specimen of the lesion was sent for microscopic examination via permanent sections. The initial biopsy was diagnosed as fibrous connective tissue with neurovascular structures, edematous muscle, adipose tissue, and salivary glands. The specimen also demonstrated focal reactive changes with fibroblasts, hemosiderin and chronic inflammation, including histiocytes, eosinophils, multinucleated cells, dystrophic calcification and extravascular hemorrhage. A differential diagnosis of reactive changes superimposed on a mesenchymal neoplasm, Langerhan cell histiocytosis, desmoplastic fibroma, intraosseous nodular fasciitis, and intraosseous inflammatory myofibroblastic tumor were considered.
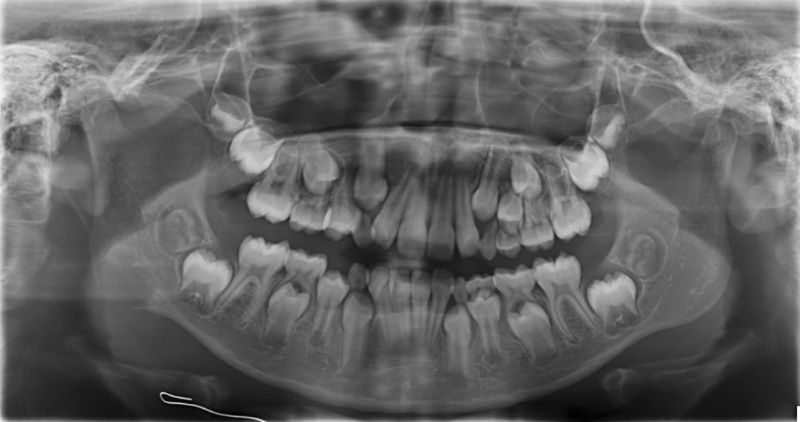 Figure 1: Panoramic radiograph at patient’s initial presentation demonstrating a radiolucent, lobulated pattern in the right mandibular angle.
Figure 1: Panoramic radiograph at patient’s initial presentation demonstrating a radiolucent, lobulated pattern in the right mandibular angle.
During the biopsy, a large amount of fluid was expressed from the lesion and minimal swelling was observed post operatively. The patient’s parents refused further treatment and the patient was temporarily lost to follow up. Six months after initial presentation, the patient returned to the office with a new CT scan demonstrating a significant increase in size of the lesion (Figure 2). The patient reported the lesion to be non-tender, non-mobile but could feel it “moving” upon mastication. Of note, the patient had no lymphadenopathy, fever, or weight loss. The patient’s mother endorsed noticing increasing tiredness and frequent naps with a new diagnosis of anemia.
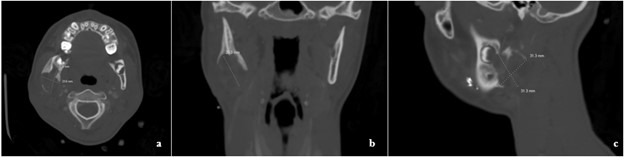
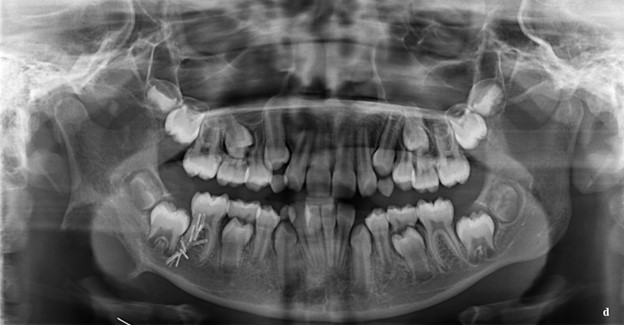
Figure 2: a. Axial, b. Coronal, c. Sagittal view of defect showing right mandibular erosion. d. Panoramic radiograph seven months after initial presentation and initial biopsy. Right mandibular defect has expanded and shows irregularity at the margins. Surgical clips present.
She was taken to the operating room for surgical removal of the lesion utilizing a right apron neck incision. Partial resection of the mandible was performed, and the resultant defect was reconstructed with a KLS Martin 3.0 reconstruction bar that was contoured, adapted, and secured (Figure 3). The gross specimen was submitted for microscopic examination. The patient tolerated the procedure well and was discharged home without complication.
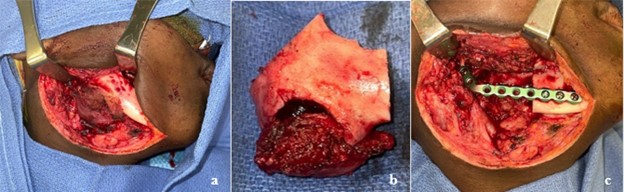
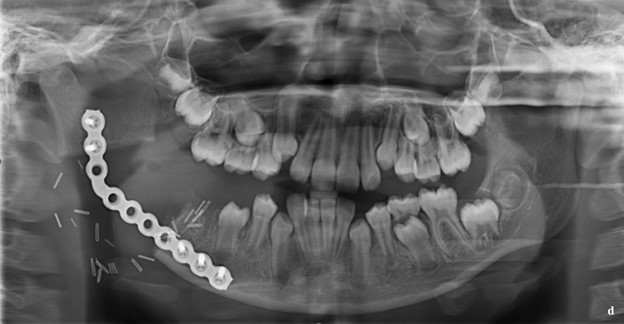
Figure 3: a. Apron incision with visualization of inferior boarder of right mandible. b. Right mandibular resected segment with appropriate margins. c. KLS Martin reconstruction plate adapted to right mandible spanning approximately 5 cm gap. d. Post-operative panoramic radiograph showing interval removal of right mandibular pathology with placement of reconstruction bar.
Gross examination revealed a “red-tan” tissue “extruding through a defect in the inferior aspect of bone.” The lesion was multinodular, soft and hemorrhagic. Microscopic examination revealed moderately eosinophilic cytoplasm and ovoid to wrinkled nuclei. No cytologic atypia or mytotic activity was found. The specimen demonstrated a syncytial growth pattern and had associated blood filled spaces and thick fibrous tissue with a lymphocytic rich infiltrate and prominent hemosiderin deposition. The pathologist noted the “mass appears to involve the medullary cavity.” Immunohistochemistry was performed which resulted in CD68 and desmin positivity. Pancytokeratin, CD45, CD163, CD31, CD34, CD68, SMA, S100, CD1a, BCL2, SATB2 and ALK1 were all negative. Next generation gene sequencing studies were not performed. The specimen was sent for expert consultation where it was described as a 2.8 cm, atypical epithelioid neoplasm with cystic change. In this report the mass was described as having cysts filled with blood and surrounded by hemosideran-laden macrophages. This second pathologist stated there was extensive lymphoid response at the periphery associated with fibrosis which they believed to be a rare example of AFH involving bone with a soft tissue extension (Figure 4).
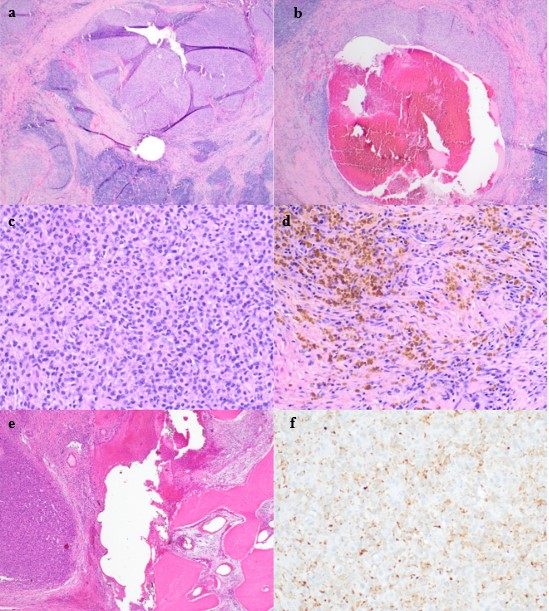
Figure 4: Histopathologic features of intraosseous angiomatoid fibrous histiocytoma. a. A multinodular tumor with a blood-filled space is surrounded by a lymphocyplasmacytic cuff and peripheral fibrous pseudocapsule (H&E stain, original magnification x 20). b. A pseudoangiomatous space containing blood is rimmed by tumor cells (H&E stain, original magnification x 100). c. At high power, the tumor is comprised of ovoid cells with bland and vesicular nuclei (H&E stain, original magnification x 200) and d. spindle shaped cells with overlying background hemosiderin (H&E stain, original magnification x 200). e. The tumor edge erodes bone (H&E stain, original magnification x 40). f. Overall, the lesion exhibited focal desmin immunoreactivity (desmin immunostain x 200). The patient has been followed closely for 15 months since submission of this report with no recurrence reported. She will continue to follow up and has been given reconstructive options.
Discussion:
Angiomatoid fibrous histiocytoma (AFH) is a rare sarcoma of soft tissues which metastasizes less than 5% of the time according to Thway et. al. [11] It is more commonly seen in children and young adults, typically affecting the extremities and trunk. [2, 5] This entity was first described by Enzinger in 1979 as angiomatoid malignant fibrous histiocytoma, a locally aggressiveness tumor with a high recurrence potential. [15] According to Rullo et. al. the World Health Organization has since designated AFH an intermediate grade malignancy. [8] Soft tissue AFH is found in the subcutaneous or deep dermal layers of the extremities or trunk. [1, 2] Shi et. al noted most masses to be painless and slow growing. [9] Most tumors are mobile, reddish-blue nodular, soft-tissue masses which can be misinterpreted as lymphadenopathy. Non-specific symptoms such as fever, malaise, anemia, weight loss may also be present. [4, 5] AFH has four distinct histopathological characteristics: 1) spindle to ovoid shaped histiocytoid tumor cells, 2) central cystic space often filled with blood, 3) a lymphoplasmacytic rim, and 4) a dense fibrous pseudocapsule. [3, 6] Most AFHs are diagnosed based on histopathology. When in doubt, immunohistochemistry, and molecular studies aid in the diagnosis. The tumor cells are positive for: CD68, epithelial membrane antigen, desmin, and CD99. [7, 8] and molecular studies show that over 90% of cases exhibit a t(2;22)(q33;q12) translocation resulting in an EWSR1-CREB1 fusion. Less common genetic abnormalities include the t(12;22)(q112;q12) translocation resulting in an EWSR1-ATF fusion and cases that harbor a FUS-ATF1 fusion. [9, 11] Our patient presented as a 9-year-old with a painless mass to the right mandible. This is consistent with Regezi et al. reporting that AFH often presents in individuals younger than 20 years of age. Ages ranged from 6 months to 83 years in our literature review with a mean age of 28.7 years (Table 1). Our patient is unique in the sense that she is female, the tumor presented itself in the head/neck region and it was intraosseous. Enzinger had shown a slight male predominance in his work and Fanburg found a ratio of 1:3, females: males, in his study of 158 cases. [7] Our literature review yielded a ratio of male: female of 49:41 in a review of 91 cases (Table 2). Lai et. al. reported AFH occurs in the extremities 60-90% of the time and in the trunk approximately 20-28% of the time. Only 5-7% of cases are in the head and neck. [6] In our review of literature, 60.4% of the 91 cases were found in the extremities, 17.6% were found in the trunk, 21.9% were noted in the head and neck (Table 3). Our review of literature focused on articles of the head and neck; potentially skewing the data in this direction.
Tables:
|
Age (yrs) |
No. of patients |
Percent of total |
|
0-9 |
24 |
26.4 |
|
10-19 |
25 |
27.5 |
|
20-29 |
18 |
19.8 |
|
30-39 |
9 |
9.9 |
|
40-49 |
8 |
8.8 |
|
50-59 |
2 |
2.2 |
|
60+ |
5 |
5.5 |
|
Total: |
91 |
100 |
Table 1: Age at time of diagnosis of AFH tumor
|
|
No. of Patients |
% of total |
|
Number of Males |
49 |
53.8 |
|
Number of Females |
41 |
45.1 |
|
Number unspecified |
1 |
1.1 |
|
Total No. Of Patients |
91 |
100 |
Table 2: Sex distribution of patients with AFH in literature review
|
Region |
No. of Patients |
% of total |
|
Head/Neck |
20 |
21.9 |
|
Trunk |
16 |
17.6 |
|
Upper Extremity |
32 |
35.2 |
|
Lower Extremity |
23 |
25.3 |
|
Total: |
91 |
100 |
Table 3: Location of AFH tumors in literature review
Compilation of data from literature review. 91 patients were included in our literature review, our patient’s data was not included in this data. Table 1: The average age of the patient at time of diagnosis. Table 2: The number of patients who were male, female and one which was not specified. 3: The locations of AFH. On gross examination of the tissue sample, our patient’s tumor was noted to be “red-tan” pigmented, nodular, and said to be hemorrhagic. In 12 of Shi et. al. cases, AFH tumors demonstrated red-brown hemorrhagic cystic spaces. Microscopically, they consisted of spindle or ovoid cells in a nodular pattern with pseudoangiomatous spaces filled with blood and had a lyphoplasmacytic rim. [9,10] The final pathologic specimen from our patient demonstrated these same classic histopathologic features of: the central cystic spaces filled with blood, the histiocytoid tumor cells, the lymphocytic cuff, and the dense surrounding fibrous tissue. For AFH, we can expect variable desmin, CD99, and EMA immunoreactivity. Desmin, epithelial membrane antigen, is positive in about 50-60% of cases according to Xiang et. al. In our case, desmin was focally positive. Other immunostains are variable and often non-specific for AFH. Additionally, all other essential histologic (H&E) criteria were present in our patient’s tumor as described histopathologically. Therefore, molecular studies confirming EWSR1 gene rearrangements were not ordered or deemed necessary.Overall, AFH is said to have good prognosis with local recurrence being around 12% and only rarely metastasizing. [7] Thway et. al. states less than 5% metastasize and describes a case of AFH of the posterior scalp that metastasized three years later to a contralateral lymph node. [11] Prognosis is negatively affected in head and neck lesions, likely due to the inability to obtain negative margins upon resection of the tumor. Costa et al. did report treatment with radiation therapy for post-surgical recurrences. Still, the majority of authors support wide surgical excision as the treatment of choice for AFH. [11, 12] We were able to achieve negative margins in our case, likely due to early presentation, diagnosis, and treatment. Our patient is now 15 months status post mandibular resection with no signs or symptoms of recurrence. She maintains regular follow up visits. Spencer et. al published an abstract in 2005 regarding a seven-year-old female who presented with an AFH in the bone of the right mandible. [14] Only an abstract exists for this case and no further documentation has been found. Apart from this, we believe there are no other documented intra-osseous AFH cases localized in the mandible. AFH is exceedingly rare in the maxillofacial region and is even more so when found in the jaws. [7,8] Chen et. al. discusses unusual locations of AFH in non-somatic tissues such as the brain, mediastinum, bone, vulva, retroperitoneum, omentum, ovary, lung and hard palate. [13] Since there are over 50 classified subtypes of sarcoma and an inability to adequately perform clinical trials on each rare subtype, case reports such as this are important in the understanding of tumor behavior, characteristics, genetics, treatment modalities and prognosis. [4]
Acknowledgements:
Our thanks to Dr. Jonathan McHugh of the Department of Pathology at the University of Michigan Hospital for the diagnosis of intraosseous angiomatoid fibrous histiocytoma. Additional thank you to Pamela Beegle at Nova Southeastern University’s Alvin Sherman Library for assisting in the literature review.
Declarations:
Ethics approval: Ethical approval was waived by the local Ethics Committee at Nova Southeastern University in view of the retrospective nature of the study and all the procedures being performed were part of the routine patient care.
Statements and Declarations/Compliance with Ethical Standards:
Author Contributions:
All authors contributed to the study conception. Dr. Shawn A. McClure performed the surgeries, followed-up with the patient after surgery and had the original idea for the article. Dr. Maria L. Nord performed the review of literature. Data collection and analysis were performed by Dr. Nord and Dr. Celis. Dr. Celis wrote the abstracts. The first draft of the manuscript was written by Dr. Nord and edited by Dr. Shawn A. McClure. Dr. Mark D. Mintline provided histopathologic review, microscopic exam of pathologic sample, expert consultation, and provided manuscript edits throughout the process. All authors read and approved the final manuscript.
Acknowledgements:
Our thanks to Dr. Jonathan McHugh of the Department of Pathology at the University of Michigan Hospital for the diagnosis of intraosseous angiomatoid fibrous histiocytoma. Additional thank you to Pamela Beegle at Nova Southeastern University’s Alvin Sherman Library for assisting in the literature review.
Anatomic Site: Right mandibular angle- intraosseous.