
Ravi K Mahavar, Ajmer Singh*, Manisha Mishra
Department of Cardiac Anaesthesia, Institute of Critical Care and Anesthesiology, Medanta-The Medicity, Sector-38, Gurugram (Haryana)-122001, India.
*Corresponding authors: Ajmer Singh, Department of Director, Cardiac Anaesthesia, Institute of Critical Care and Anesthesiology, Medanta-The Medicity, Sector-38, Gurugram-122001, India
Received Date: January 18,2024
Accepted Date: January 24,2024
Published Date: January 30, 2024
Citation: SMahavar Ravi K, Singh A, Mishra M (2024). “Unicommissural Unicuspid Aortic Valve: A Case Report”. Clinical Research and Clinical Case Reports, 5(1); DOI: http;//doi.org/08.2024/1.1073.
Copyright: © 2024 Ajmer Singh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Unicuspid aortic valve, a rare congenital anomaly of the aortic valve, generally manifests between the 3rd to 5th decade of life. Symptoms can be related to aortic valve stenosis or regurgitation. Transesophageal echocardiography is the mainstay of the diagnosis. We, herein, report a case of a 17-year-old patient who was admitted with the symptoms of aortic valve stenosis and was diagnosed to have unicommissural unicuspid aortic valve. Compared to the patients with bicuspid aortic valve, these patients are at higher risks of aortic dissection, infective endocarditis, and the need for pacemaker implantation.
aortic valve; echocardiography
Introduction:
Unicuspid aortic valve (UAV), a rare congenital anomaly of the aortic valve, can result in aortic stenosis, aortic regurgitation, or both. It should be differentiated from the bicuspid aortic valve (BAV), which is a more common abnormality. Both UAVs and BAVs can lead to premature calcification of the valve, dilation of aortic root, or aortic dissection.1 In unicuspid valves, these physiological and pathological changes occur rather expeditiously. We here in, report a case of a UAV along with relevant transesophageal echocardiography (TEE) images.
Case Report:
An otherwise healthy 17-year-old boy came to our institution with complaints of exertional dyspnea, on and off chest pain, lightheadedness, and dizziness. The physical examination showed a midsystolic ejection murmur heard at the right heart border. Preoperative transthoracic echocardiography (TTE) showed a calcified UAV with severe aortic valve stenosis and trace aortic regurgitation. The transvalvular mean/peak gradients were 47/72 mmHg, the velocity time integral (VTI) ratio was 0.2, and the aortic valve area by continuity equation was 0.8 cm2. Other dimensions were as follows: aortic annulus 2.1 cm, aortic root 2.8 cm with normal sinuses of Valsalva, and ascending aorta 3.8 cm. The left ventricular ejection fraction was 60% with moderate concentric ventricular hypertrophy. After obtaining informed consent for the aortic valve replacement surgery, intraoperative TEE was performed which confirmed the preoperative TTE findings along with detection of a patent foramen ovale of 3 mm size with a left to right shunt. A unicommissural UAV was seen, and the commissure was located between the noncoronary and left coronary cusp, along with a characteristic eccentric “tear-drop” orifice pointed posteriorly (Figure 1, Video 1).
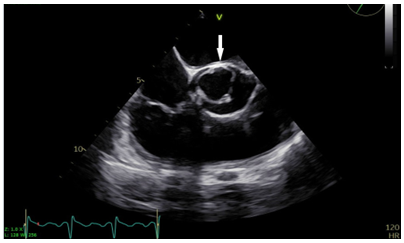
Figure 1: Transesophageal aortic valve short-axis view showing unicommissural unicuspid aortic valve in systole, with commissural attachment at 12 o’clock position (arrow)
Video 1: Transesophageal aortic valve short-axis view showing unicommissural unicuspid aortic valve.
During surgery, the calcified valve was excised and replaced with a 21 mm SJM mechanical valve (St Jude Medical, Minnesota, USA). Patent foramen ovale was closed using a 3-0 Prolene suture. The remaining course of the patient in the hospital was uneventful.
Discussion:
The UAV, first published by Edwards in 1958, is a rare congenital abnormality of the aortic valve with an incidence of 0.02% in patients undergoing echocardiographic screening and approximately 4-5% in patients undergoing aortic valve surgery.2-4 It has two subtypes depending on the presence or absence of a commissure: acommissural and unicommissural UAV with some characteristic differences. Acommissural UAV, as the name suggests, does not have a commissure. There is a “pinhole” orifice with no lateral attachment to the aorta. The acommissural UAV usually manifests at birth or during infancy due to symptoms caused by severe aortic stenosis. Many patients with acommissural UAV need intervention during their infancy. On the other hand, the unicommissural subtype has a single commissure with a lateral attachment to the aorta, along with an eccentric “tear-drop” orifice. The most common site of commissural attachment is between the noncoronary and left coronary cusp as observed in this patient.2 The BAV, on the contrary, has two commissures, two cusps, and an elliptical “fish mouth” appearance. A multi-detector computed tomography may prove useful when faced with the dilemma of differentiating UAV from BAV due to excessive calcification. UAV most commonly manifests as aortic stenosis, and less often as aortic regurgitation. The usual age of presentation is the 3rd to 5th decades.5 The most common symptoms in adults are angina, exertional dyspnea, dizziness and/or syncope, etc. In the pediatric population, the patients may present with failure to thrive and heart failure. UAV may be associated with other disorders like aortopathy, ventricular septal defect, anomalous coronaries, and patent ductus arteriosus.6 The aortopathy, resulting from concomitant effect of hemodynamic stress and weakened aortic media, can lead to coarctation of the aorta, aortic dissection, or formation of an aortic aneurysm. Compared to the patients with trileaflet aortic valve, the risk of aortic dissection in patients with UAV and BAV is 18 and 9 times higher respectively. In addition, the UAV patients are at a higher risk of infective endocarditis due to high turbulence around the valve. Furthermore, the patients with UAV are at a potentially higher risk of need for pacemaker implantation as the valvular calcification could extend into the interventricular septum with the likelihood of injury to conduction system during debridement.The management options in patients with UAV with some salient drawbacks include: (i) aortic valve replacement using bioprosthetic valve: risk of early structural degeneration and need for re-operations, (ii) replacement using mechanical valve: risk of bleeding or thrombosis. (iii) Ross procedure using a pulmonary autograft: failure of autograft, though superior long-lasting results in younger patients, (iv) aortic valve repair or Ozaki procedure: poor replication of results, premature degeneration of the patch, suture dehiscence or endocarditis, (v) transcatheter aortic valve implantation: risk of paravalvular leak, increased need for pacemaker implantation, difficult coronary access following intervention, cannot address concomitant aortopathy.7 Differentiating UAV from the BAV is important in the era of transcatheter intervention. While the indications for transcatheter intervention are increasing in selected patients with BAVs, the authors have not come across any such reports in patients with UAV, however. In conclusion, UAV, an infrequent cause of aortic valve stenosis, should be a part of the differential diagnosis of aortic stenosis in younger patients.