Kulvinder Kochar Kaur 1*, Gautam Allahbadia 2, Mandeep Singh 3
1 Scientific Director, Kulvinder Kaur Centre For Human Reproduction, 721,G.T.B. Nagar, Jalandhar-144001, Punjab, India
2Scientific Director, Ex-Rotunda-A Centre for Human Reproduction, 672,Kalpak Garden,Perry Cross Road, Near Otter’s Club,Bandra(W)-400040 Mumbai, India
3Consultant Neurologist, Swami Satyanand Hospital, Near Nawi Kachehri,Baradri,, Ladowali road,JALANDHAR, Punjab India.
*Corresponding author: Kulvinder Kochar Kaur, Scientific Director, Kulvinder Kaur Centre For Human Reproduction, 721,G.T.B. Nagar, Jalandhar-144001, Punjab, India
*Corresponding author: Kulvinder Kochar Kaur, Scientific Director, Kulvinder Kaur Centre For Human Reproduction, 721,G.T.B. Nagar, Jalandhar-144001, Punjab, India
Accepted date: February 09, 2021
Accepted date: February 18, 2021
published date: February 24, 2021
Citation: Kulvinder K Kaur, Allahbadia G, Singh M. “Are we Any Close to Unraveling the Mechanism of Interactions Among Susceptibility Genes Towards Type 1 Diabetes, Gut Microbiota Along with Environmental Factors , Specifically Early Diet Patterns –A Systematic Review.’’. Endocrinology and Surgical Endocrinology, 2(1); DOI: http;//doi.org/03.2021/1.1005.
Copyright: © 2021 Kulvinder Kochar Kaur. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Earlier wehad reviewed on the aetiopathogenesis of Type 1 diabetes mellitus(T1D) along with role of gutmicrobiota(GM) ,genes ,immunotherapies besides the role ofGM in obesity ,type 2 diabetes and probiotics in detail. Whereas the pathogens for autoimmune diseases continue to be mostly not clear ,genetic proneness as well as environmental factors have been believed to be the main etiologies.Of the environmental factors the, microbiome is associated with autoimmune diseases through direct as well as indirect crosstalk with innate as well as adaptive immune cells .This leads to loss of immune tolerance ,chronic inflammation as well as immune response against host tissues .The posited part of microbiome in autoimmunity are Molecular mimicry ,epitope spreading ,bystander activation, as well as continued infection.Further the longitudinal studies have pointed toward the implication of geographical variations .Here we decided to conduct a systematic review on the role of gut microbiota and its relation with Type 1 diabetes mellitus,interaction with other environmental factors like delivery mode ,mode of baby feeding and its impact on GMlike use of breast feeding only at least till 4 mths ,Ultimately it has been observed that delaying gluten introduction till 4mths as well as cows milk beyond 12mths of age along with addition of early pre/probiotics in those children possessing high risk susceptibility genes .More work is required to evaluate gut virome and other components like archeome ,Microbiota of vagina ,skin as well as metabolome to arrive at a conclusion .Moreover use of diets like Mediterranean diet ,FUN2 as well as ArH targeting to avoid generation of T1D needs to be exploited.
1. Introduction
Earlier wehad reviewed on the aetiopathogenesis of Type 1 diabetes mellitus(T1D) along with role of gutmicrobiota(GM) ,genes ,immunotherapies along with role ofGM in obesity ,type 2 diabetes and probiotics in detail[1-12] .The human microbiome comprises of trillions of bacterial ,viral as well as fungal microorganisms[13].Coevolution took place in a symbiotic fashion along with humans for 100-1000’s of years[14].Why so much variation of these microorganisms occurs is secondary to host lifestyle ,geographical placement ,dietary habits,infections ,sex,age as well as genetic background[15].It has been demonstrated that the human microbiome has the capacity to influence the host physiology in multiple ways like metabolism , immunity , along with behavior[16].Thus interfering with human microbiota might cause disease.Multiple studies have ,illustrated that the human microbiome has the capacity to influence the etiopathogenesis of immune diseases, Specifically autoimmune diseases,where the immune system has the inability of differentiating self from the nonself proteins as well as attack self tissues .Like in Multiple Sclerosis (MS)[16], Rheumatoid arthritis( RA), systemic lupus erythematosus(SLE), antiphospholipid syndrome( APS)[17], Crohn’s disease(CD) [18], Ulcerative colitis (UC)[19], inflammatory bowel disease (IBD)[20].Coeliac disease[12], Type 1 diabetes mellitus(T1D) [22,23].
The existence of particular Gut Microbiota(GM) as well as total diversity ,are key in generation of the nascent immune system.Studies carried out in germ free( GF) as well as gnotobiotic mouse models demonstrated significant part in the manipulation as well as differentiation of innate immune cell kinds particularly interleukin(IL)-17 Generating CD4+T cells (Th17cells) as well as Foxp3 + regulatory T(Treg)cells[24]. Particularly segmentous filamentous bacteria(SFB) are believed to stimulate the expression of pro inflammatory Th17cells, significant for sustaining the mucosal barrier as well as , confer protection to non obese diabetic(NOD) mice from Generating T1D[24,25]. Nevertheless,in other mouse models of autoimmune diseases(like K/BxN mouse models OF autoimmune arthritis ). SFB are documented to facilitate disease propagation through significant Th17 collection ,pointing to their part in autoimmunity is etiologically particular [26].Other bacteria confer protection like Lactobacillus, Bifidobacterium as well as Clostridium species,are responsible for the stimulation of ant- inflammatory Treg)cells whereas Bacteroides fragilis polysaccharide A(PSA) stimulates IL-10 generation as well as is understood to repress Th17cells responses[27].
Whereas the pathogens for autoimmune diseases continue to be mostly not clear ,genetic proneness [28] as well as environmental factors [29] have been believed to be the main etiologies.Of the environmental factors the, microbiome is associated with autoimmune diseases through direct as well as indirect crosstalk with innate as well as adaptive immune cells .This leads to loss of immune tolerance ,chronic inflammation as well as immune response against host tissues [30].The posited part of microbiome in autoimmunity are Molecular mimicry ,epitope spreading ,bystander activation, as well as continued infection[30,31].Hence the immune tolerance loss might get stimulated changes in microbiome composition .Various studies have actually illustrated that the gut microbiome composition of patients with autoimmune diseases is markedly separate in contrast to healthy subjects[32-35].
Leaving few exceptions (like Sardinia ,Italy ), autoimmune diseases incidence keeps a north-south wave ,with escalated incidences observed in Nordic countries ,like Finland,Sweden as well as Norway [36,37].GM studies on the influence of extreme climatic situations (like polar expeditions )[38] as well as birth month/place [39] reveal how climate, Specifically sunlight exposure ,influence GM composition as well as immune impairment.The factors that are significant in northern population are circadian rhythm impairment as well as Viamin D deficiencies ,that have also been illustrated to lead to immune impairment through a swing in the GM ,resulting in autoimmune diseases like T1D[40,41].
T1D is believed to be a disease having the properties of insulin deficiency secondary to autoimmune injury or function loss of the pancreatic insulin generating beta cells .It is significant to observe that autoimmunity is found as a major factor resulting in T1D by most researchers ,certain workers believe that autoimmunity occurs following other factors like endoplasmic reticulum(ER) stress resulting in beta cells deletion[42].By historical facts T1D has been believed to be escalated blood glucose amounts(hyperglycemia), as well as the existence of 1 or greater antibodies,all of which take place/or are existent prior to beta cells ablation[43]. Autoimmunity can get mounted against insulin(IAA), glutamic acid decarboxylase((GAD 65),Insulinoma associated autoantigen-2(IA2A), as well as/or zinc transporter8(ZnT8A) along with may occur many years before symptoms onset[43].Besides the major antibodies,a new found family of neoepitopes Generated with post translation modulation got isolated[44].Hybrid insulin peptides(HIP’s) represent a very intriguing neo peptides which get generated with the fusing of an insulin neopeptide along with a liberating granule peptides in the granules[45].The commonest autoantibody seen before the disease initiation is against IAA,with the IAA amounts association robustly with the rate of propagation towards overt T1D in children [43].This event is usually known as seroconversion ,a significant terminology utilized all through this review.Here we decided to conduct a systematic review on the role of GM in detail with regards to T1D .
Methods
Thus a systematic review was carried out using the pubmed, Web of Science , Medline, Embase, Cochrane reviews, and Google Scholar, Search engine with the MeSH Terms; impaired lipid metabolism; oxidative stress;inflammation; ;Gut Microbiota(GM); Type 1 diabetes (T1D);breast feeding ;mode of delivery;gluten foods introduction;role of omics in studying the etiopathogenesis of T1D from 1990’s till date in 2021.
Results
We found a total of 750 articles, out of which we selected 194 articles for this review.No meta-analysis was done. did a search using the pubmed search engine using the MeSH terms
Whereas T1D remains 1 of the maximum represented chronic ailments in childhood ,approximately 25%of individuals diagnosed with this disease compromise of adults [46].There has been an escalation of the incidence worldwide for the past few decades particularly post World war II in the west [47-49].Although great genetic impact is there the enhancement of T1D prevalence as well as varying incidence rates across various countries , as well as even among countries that are just adjacent to each other in Europe(Russia Karelia as well as Finnish Karelia),points to an interaction among proneness genes along with particular environmental effects[50].Discordant outcomes for T1D twins ,all point that environmental effects have a great part in the etiopathogenesis of the disease[51].
The non-obese diabetic(NOD) mouse model is the commonest animal model utilized to study T1D,in view of considerable akinness to human T1D regards to recognition of autoantigens,immunopathology as well as gene proneness[52].Akin to humans in NOD) mouse model shows T1D correlated variation in MHC Class II that influences the presentation of islet –obtained antigens to the T Cells .Besides that ,a lot of non MHC prone genes that are common both for NOD mice as well as humans are correlated with T1D risk like protein tyrosine phosphatase non-receptor22(PTPN22)gene, of cytotoxic lymphocyte associated protein 4 gene(CTLA4), IL2RA(codes-αsubunit of IL2R[53].It was initially seen that colony hygiene influences the incidence of T1D in NOD mice [54].Other studies on NOD mice have further illustrated that the Gut Microbiome can crossreact with immune system to control DM pathogenesis in mice [55]. Nevertheless,unlike humans ,female NOD mice possesses significantly greater T1D incidence in contrast to male NOD mice.A study conducted by Markle etal.,[56] revealed that male NOD mice in specific pathogen free(SPE) situations were greater protected against T1D in contrast to female NOD mice. Nevertheless, in germ free situations, both male as well as female mice had equal incidence rates.Some commensal bacteria that are believed to escalate T amounts are also observed to have the capacity of protecting male NOD mice against T1D onset [56].Intriguingly ,transfer of GM from male NOD mice to female mice changed the composition of Gut Microbiome in recipient mice and hence protected them from T1D [56].This points that changing of Gut Microbiome can implicate immune system as well as pathogenesis of disease .
Inspite of variations in NOD) mouse model T1D etiology that is apparent,studies conducted in vivo give the mode of action strategy that is not feasible in human studies having been key in getting insight in disease onset as well as propagation.Here longitudinal studies as well as those on NOD mice along with role of Gut Microbiome as it associates to environmental factor in T1D pathogenesis is detailed.
2.Human Longitudinal Studies
Genome –wide association study (GWAS) studies have observed equivalent to 50 genetic areas which implicate the risk of generation of T1D[57].The risk of generation of T1D gets decided by finding the genetic factors like T1D-correlated SNP in the human leukocyte antigen (HLA )gene ,more particularly ,the HLA-DQ as well as HLA-DR protein coding genes DQ1 as well as DQB1[58]. Nevertheless, just genetic proneness is not enough to reason out the T1D onset[59].Till now the outcomes from these studies have been wide as well as primarily associative ,pointing to the multiple facets of this disease.
For finding the etiopathogenetic environmental factors that initiate the disease origin, longitudinal studies of huge at –risk cohorts are needed across a broad geographical area.These actions have got started –are Teddy , DABIMMUNE ,BABYDIET(i.e a substudy of the huge BABYDIAB),ABIS,TRIGR as well as FINDIA(a substudy in the Type 1 diabetes mellitus Prediction as well as Prevention DIPP Study).Here observations of Teddy , DABIMMUNE as well as ABIS,is detailed with concentration on Gut Microbiome in the generation of autoimmunity .
2.1Teddy Study
The environmental determinants of diabetes in the Young(TEDDY)got designed for constantly monitoring the children isolated having a proneness for generation of T1D.The study got carried out in 6 various clinical centers in Colorado ,Washington State,Georgia/Florida in the US as well as Finland ,Germany along with Sweden.From 2004-2010 ,general population(GP) along with first degree relations(FDR)newborns got screened for HLA kinds[58].Regarding all the 6 geographical areas, same high risk haplogenotypes got isolated,of these haplogenotypes were taken into account for the inclusion particulars for the GP(DR3/4, DR4/4, DR4/8, DR3/3),whereas 9 haplogenotypes were taken into account for the FDR(DR3/4, DR4/4, DR4/8, DR3/3, DR4/4b, DR4/1, DR4/13, DR4/9 along with DR3/9((https://teddy .epi.usf .edu/research).
2 extra studies got carried out utilizing TEDDY samples.In the study by Vatanen etal.[23],stool samples got acquired as well as sequenced from 783 children mthly at age3mths till the clinical end stage (seroconversion , autoantibodies getting found ).In islet autoimmunity(IA),case control cohorts had a greater prevalence of Streptococcus group/mitis/oralis /pneumonia whereas controls possessed greater prevalence Lactobacillus rhamnosus as well as Bifidobacterium dentium ,2 usually species in probiotic cocktails.In T1D case control cohorts , T1Dcases possessed greater amount of Bifidobacterium pseudo catenulam,Roseburia hominis , as well as Alistepes shahii, whereas healthy controls possessed greater ,Streptococcus thermophilus as well as Lactobacillus lactis .Stewart et al.,[60]in another study evaluated stool samples from 903 children among 3 as well as 46mths of age utilizing 16S rRNA (V4) as well as carried out metagenomic sequencing results.A nested case control Evaluation documented that alpha diversity Microbiata maturation as well as Microbiata by age Z scores(MAZ) were akin among cases as well as controls regards to both IA as well as T1D cohorts .The relative excess of maximum escalated genera illustrated subtle composition changes that had a greater prevalence of Erysipelotrichaceae in IA cases .In contrast to healthy controls, T1D cases possessed a greater excess of Parabacteroides(p<0.001) as well as a reduced prevalence of11 genera of Ruminococcacea, Lactococcus, Streptococcus as well as Akkermansia.In total TEDDY isolated various bacteria weakly associated with T1D initiation, Nevertheless,future studies are required for finding out the mode of actions working.
2.2 Dabimmune Study
In case of northern Europe , greater variation in incidence of autoimmune diseases have been demonstrated among adjacent countries .Like despite the frequency of HLA genotypes are quite akin among Finland as well as Russian Karelia ,the incidence of T1D are 6times greater in Finland[60].These trends seen are believed to be based on the national public healthy standards along with personal hygiene methods, as well as seen that it is the developed countries having a greater incidence of autoimmune diseases like T1D in contrast to lesser developed countries.Hygiene as well as autoimmunity ( like Hygiene posit ) are correlated with the found reduction in GM diversity in greater hygienic surroundings[61-63].The absence of alpha diversity is believed to escalate the chances of pathogenic invasion (like antibiotic stimulated Clostridium difficile infections )[64] as well as has been demonstrated to accelerate autoimmunity in persons prone to same. The DABIMMUNE longitudinal study tried to isolate the environmental factors which could be pointed to have greater chances of autoimmune as well as allergenic diseases.
In 2008,in DABIMMUNE, recruitment of equivalent to 1000 newborn infants possessing high risk HLA haplotypes from Finland,Estonia as well as Russia was done.Blood In addition to stool samples as well as clinical metadata ,got acquired from 1mth to 3yrs of age .The earlier Evaluation got published in 2015 that concentrated on Finnish as well as Estonian candidates[65].In this study a cohort of 33 infants who had genetic proneness to T1D.Of this cohort ,11 children generated auto antibodies(i.e seroconverted), as well as of the11 seroconverted candidates,4 of them generated T1D. Microbiome Evaluation were carried out utilizing 16S rRNA along with metagenomic shotgun sequencing data.In total this study observed reduction in Microbial diversity as well as decrease in bacterial gene amount in auto antibody –positive children during propagation towards T1D. Particularly they observed a reduction in Lachnospiraceae as well as Veillonellaceae in children generated T1D besides an escalation of Streptococcus,Ruminococcus, as well as Blautia(figure1)[66].
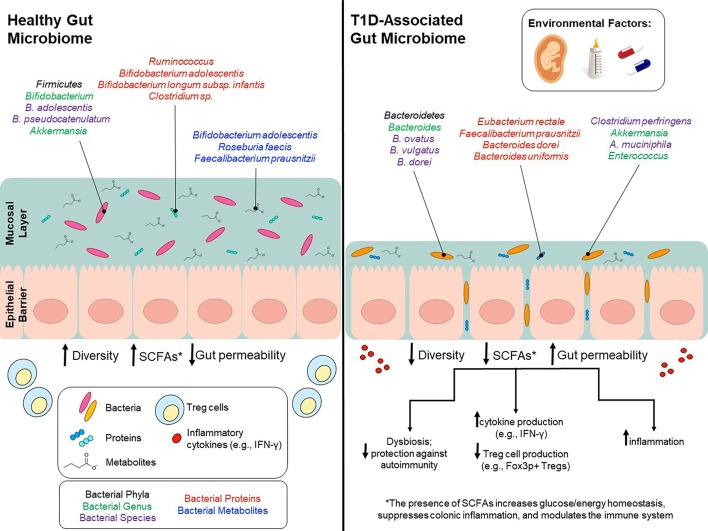
Legend for Figure 1
Courtesy ref no-67-Environmental factors modulate gut microbiota and potentially contribute to T1D onset. Environmental factors, such as birth mode, diet early in life, and use of antibiotics can influence gut microbiota composition and can to lead to lower bacterial diversity, decreased SCFA production and increased gut permeability. Bacterial phyla/genus/species that are affected by environmental factors and differ between T1D patients and healthy controls are depicted in the colors black/green/purple, respectively. Bacterial genus/species that have been identified in proteomic analyses and are increased in either T1D patients or healthy controls are shown in red. Bacterial genus/species that have been identified in metabolomics analyses and are increased in either T1D patients or healthy controls are shown in blue.
A functional Evaluation observed that bacterial metabolism in auto antibody –positive candidates had the properties of a greater prevalence of genes implicated in transport of sugar as well as lower prevalence of genes implicated in amino acid generation in contrast to those that did not have seroconvertion.
In a consequent DABIMMUNE study[67],a metagenomic Evaluation of222 Finnish, Estonian, as well as Russian children(with subcohorts of 74 children from every country),that were matched for HLA risk as well as gender ,observed no association among islet auto antibodies or T1D status along with Microbiome composition. Nevertheless,these authors documented variable escalation of Bacteroides species(spp). Bacteroides species(spp),had lower excess in Russian children in contrast to children from adjacent countries like Finland as well as Estonia.They posited that the greater prevalence of T1D in Finland as well as Estonia were correlated with early life lipopolysaccharide (LPS) exposure. lipopolysaccharide (LPS) exposure would potentially arise from Escherichia Coli (E.Coli) for Russian children in contrast to children from adjacent countries like Finland as well as Estonian who would most probably have early life exposure to Bacteroides species(spp’LPS.
Just like TEDDY, DABIMMUNE study observed variations in GM composition along with total reduction in microbial diversity in T1D subjects.This study further illustrated a potential mode utilizing , in vitro experiments that explored the structural as well as functional properties of Bacteroides dorei LPS in contrast to E.Coli LPS.
2.3Abis Study
All babies in southeast Sweden(ABIS)represents a prospective study that aims to evaluate the birth cohort for the etiopathogenesis of immune –mediated diseases,in particular T1D[68].All the mothers belonging to southeast Sweden who delivered a live child between October 1997- October 1999 were requested to take part .Overall equivalent to 17,000 babies took part in the study (78.6% born in this particular area ), as well as till date 147 of these went on to generate T1D.From these newborns blood,stool along with other biological samples were taken at1mth,2.5-3,5-6.8, as well as 11-12 yrs of age .In a study conducted recently from this Abis cohort,the authors evaluated the association among HLA haplotype along with its action on GM composition[25].Earlier work in mice had revealed ,an inability of presentation of class II antigens/ as well as /or separate major histocompatibility complex (MHC) class II haplotypes causes variations in GM composition[25,69].In a study, the absence of class II antigen presentation resulted in reduction of lactobacillus spp as well as escalation of SFB[69].In the Abis Study ,it was observed by Russell etal.[25],that results akin to mice in humans having a greater genetic chances of generation of TID autoimmunity got correlated with changes in the GM composition .They realized that the core GM composition as well as the beta diversity varied on the basis of HLA risk group as well as total genotype. Furthermore some protective HLA haplotypes got associated with the genera Intestinbacter as well as Romboutsia [25].Another intriguing study evaluated the association among pets exposure along with TID.They observed that 45.5% of pregnant women enrolled for the study possessed pets .Commonest were cats as well as dogs ,although exposure to either cats or dogs was not correlated to TID chances[70]. Nevertheless, they observed exposure to hamsters significantly enhanced TID chances.This was an intriguing observation since a common study dependent on the Canadian Health Infant Longitudinal Developmental Study(CHILD) demonstrated that early exposure to household pets change the GM composition in the infants might decrease the chances of some of diseases like obesity as well as allergic diseases[71].
3.Influence of environmental factors on Microbiata GM as well as T1D
In between birth as well as age3,the Microbiata of infants alters at a dramatic pace in view of it getting continuous exposure to innovative environmental /maternal Microbiata along with food/animal born antigens.By the age 3 ,these oscillations reduce once the Microbiata composition settles to that of the adult –like situation[60].Early life factors,like mode of delivery ,breast fed or not, cow’s milk exposure as well as a getting introduced to solid foods have been illustrated to implicate the early life microbiata composition,thus deciding its ultimate composition.Hence Early life factors have their impact beyond infancy as well as a most probably implicate health along withgeneration of diseases later in life .
3.1. Method of Delivery
Earlier work implied that the uterus represents a sterile environment,totally lacking bacteria. Nevertheless, recent research have queried this.It has been seen in a lot of studies that at the time of intrauterine period of growth ,the fetus gets exposure to maternal Microbiata through transplacental passage into the amniotic fluid[72].Prepartum ,unidirectional transfer in the maternal GM among the 1st as well as 3rd trimester possibly affected by hormonal along with immunological alterations at this time period [73].This is believed to decrease at this period of time .Intriguingly ,this switch enhances the butyrate generating taxa which facilitates escalated amounts of immunomodulatory regulatory T(Treg)cells[73].This is believed to decrease the probability of maternal rejection of the fetus[74].A 2nd switch in GM is seen just before birth.This switch results in greater heterogeneity , decreased alpha diversity , along with over expression of taxa believed to stimulate inflammation[74,75].Work conducted in humanized germ free( GF) mice has pointed that these alterations are adaptive as well as facilitate escalated energy shift among the mother as well as fetus [75]. These taxa are further thought to facilitate the proper colonization of anaerobic spp which are predominant in the neonates early life microbiata .The mother’s vaginal microbiata also go through changes just before delivery .At the time of pregnancy 4 main Lactobacillus spp escalate markedly as well as aid in stability of this community while also decreasing the alpha diversity[74].These dominant taxa don’t have a major part in sustaining vaginal pH or in avoidance of infection,but rather are believed to escalate secondary to their significance in original neonatal colonization[74].
3.1b Delivery Mode as well as part in GM Manipulation
Lot ofstudies have associated delivery mode with clear cut differences in neonatal microbiata[76,77]. association among delivery mode as well as enhancement of chances of obesity , asthma,allergic as well as autoimmune diseases[77-79]. Overall the proof robustly points that delivery mode has a massive influence on microbiata composition as well as succession.Those infants that had a vaginal delivery possess a microbiome quite akin to the vaginal microbiome of the mother whereas LSCS delivered babies get classically colonized with the species observed on the mothers skin[77].Variations in the taxa are maximum separate in the 1st 3 mths of life .Those having a vaginal delivery possess commensal bacteria like Lactobacillus as well as Bifidobacterium , whereas LSCS delivered babies are colonized with Clostridium spp as well as Staphylococcus spp[77]. TEDDY further observed that children Subsequent to a vaginal delivery possessed greater Bacteroides amounts ,that in turn was associated with a greater amounts of diversity as well as rapid maturation of the GM[60].the initial stages of microbiata exposure/colonization finally decides the composition as well as succession of the microbiata of the infants along with effects the total metabolism along with immunomodulation .Hence ,initial colonization by abnormal microbiata can cause longterm impact on the immune function along with can enhance the child’s chances of generation autoimmune correlated diseases,like TID.Various studies have observed that the colonization of Lactobacillus, Bifidobacterium along with Bacteroides gets delayed or is totally lacking in infants that are LSCS delivered[76,77,80,81] as well as absence of exposure to, Lactobacillus which gets derived from the vaginal that can result in variation in microbiata succession.Later diversity for the main phyla of GM-Actinobacterium(predominantly Bifidobacterium) Proteobacteria as well as Bacteroides-is further lesser in infants that are LSCS delivered[75,81-83].
3.2 Delivery Mode as well as part in T1D Initiation
In a meta-analysis conducted by Cardwell et al. contrasted the outcomes of 20 separate studies to find if chances of T1D associated with Delivery Mode. Following adjustments for covariates like birth weight,gestation period,maternal age , maternal T1D incidence , as well as breastfeeding there was still a 20% escalation of T1D initiation in infants that are lower segment caesarean section (LSCS )delivered[83].Still considering the individual outcomes of studies shows a lot of discrepancies. Various studies have seen a significant association among Delivery Mode as well as part in T1D occurrence [84,85], whereas others have shown little to no link[84,85].
Work regards to a direct association with delivery mode, microbiota as well as T1D incidence is minimal, however proof till date points that infants that are LSCS delivered as well as abnormal Microbiota has an impact on both T1D initiation along with propagation .In a study involving NOD mouse,investigators observed particular variations in Microbiota composition among pups that were LSCS delivered as well as those having a vaginal delivery,though they couldn’t observe a significant correlation among delivery mode as well as T1D incidence[86].They further found lesser amounts of Foxp3 + regulatory T(Treg)cells, anti-inflammatory IL-10 as well as tolerogenic CD103+DCs,pointing that LSCS delivery possesses longterm actions which probably result in impaired immunosuppression , thus escalating the chances of generation of anti-islet autoimmunity[86].
Further Evaluation of particular genera that have been believed to Initiate the onset of disease shows dramatic variations in microbiota composition as well as irregularities in the part that is played by Bacteroides spp. Bacteroides has a significant part in total immune system generation as well as function by stimulation of plasmocyte generation of secretory IgA[84,87].More particularly ,B.thetaiotaomicron is implicated in sustaining the gut barrier whereas B.fragilis as well as B.subtilis have been demonstrated to facilitate gut correlated lymphoid tissue maturation as well as work in pre immunity antibody generation [84,88]. B.fragilis further inhibited the pro inflammatory cytokine IL-17 in the intestine [84,89].
On the other hand one of the maximum observed differences in microbiota for the seroconverted high risk children was the escalated Bacteroides amounts. An example is the longitudinal Type 1 diabetes mellitus Prediction as well as Prevention( DIPP) Study,which is a Finnish research work that Initiated blood, along with stool samples from children possessing high risk HLAgroup genotype in 1994[90].A study that was more recent, utilized 4 matched case control pairs from DIPP to isolate Microbiota that varied among cases as well as controls[91].Depending on their Evaluation,one main difference was the excess of Bacteroides as well as Firmicutes . Cases possessed a significantly greater escalation of Bacteroides,that enhanced over time in contrast to controls .Validating these outcomes ,of the taxa isolsted in the DABIMMUNE study , Bacteroides spp were observed to be maximum in excess in the seroconverted cohorts from Finland as well as Estonia[91]. Studies akin to this observed that Bacteroides spp got overexpressed in case subjects, particularly the spp Bacteroides ovatus ,that was implicated for 24%of the escalation found[91].A study that was more current Finnish one[92] validates these observations utilizing 76 at high risk children that they monitored from birth to 2 yrs of age.Metagenomic Evaluation isolated 2 spp B. dorei as well as B.vulgatus ,which were significantly greater in cases in contrast to controls before seroconversion[92]. Nevertheless, a lot of Studies have arrived at separate conclusions or have observed no association at all among Bacteroides escalation as well as T1D Initiation[93].Figure 1 shows a summary of these observations as well as the variations in the microbiata composition of T1D patients along with controls.
3.4 Role of Breast feeding
Lot of work has observed the advantages that Breast milk yields for an infants growth as well as generation[94-96.Bioactive agents possessed by Breast milk are antimicrobial as well as immunomodulatory components ,have got demonstrated to modulate the GIT as well as immune function,beside GM composition via multiple modes of action.[94-96].
3.4b Role of Breast feeding in Microbiota Manipulation
Besides Bioactive agents,the maternal microbiata gets shifted in Breast milk as well as alters infants GM that are associated with the rate of breast feeding in a dose-bases way[97]. Particularly ,lactic acid bacteria in the genera Bifidobacterium as well as Lactobacillus,possessing the ability of breaking down human milk oligosaccharides(HMOs),get shifted from the mother towards the child[98].These genera are believed to sustain the intestinal barrier ,induce the generation of IgA antibodies as well as gets implicated in the generation of short chain fatty acids( SCFAS)([99-102].Noticably ,the spp Bifidobacterium longum subspp infantis gets excessive in infants which received only breast feeding strictly for1st 6mth of life[60].B. infantis is Specifically efficacious in the metabolism of HMOs into SCFAS(indirectly) along with has the ability to facilitate mucus generation,ameliorate diet induced colonic mucus degradation along with possess significant part in immunomodulation[103](figure1).
3.4c Role of Breast feeding as well as part in T1D Initiation
For the immunologic generation successfully crosstalk among host along with its Microbiota at the mucosal surface of the intestine needs to occur [102,104].In murine studies key generational windows where microbe –driven immune-control can take place .Similarly in humans it holds true –detailed later in gluten dependent foods .Thus these results point that the existence of Microbiota along with the time of these microbiota get introduced are crucial for the appropriate immune system generation.For those children who have genetic proneness to T1D, Breast milk is believed to possess protective characteristics against T1Dgeneration[105].Furthermore ,infants who are only receiving Breast feeding during the initial 6mths of life have a separate microbiota composition .It has been posited that these protective characteristics of Breast milk are efficacious by manipulation of GM composition[106-108].
In 2015 ,MIDIA,a Norwegian study Evaluating the correlation among Breast feeding time period, age at which solid foods get introduced , as well as the chances of islet –autoimmunity/ T1D in children possessing genetic proneness to T1D observed that any particular frequency of Breast feeding for12mths or greater had an association with a slower propagation towards as well as total reduction in T1D incidence [109]. Nevertheless, both age of solid foods introduction,or if infants were getting Breast feeding at the time of introduction were seen to have any action on islet –autoimmunity as well as or T1D propagation[109].These outcomes point that Breast feeding by itself possesses having a significant influence on T1D propagation/ Initiation,whereas the introduction of other food sources ,like solid foods ,might not have a significant influence[110,111].
Most of the studies conducted have concentrated on the Firmicutes :Bacteroides ratio that is a potential indicator for the generation of disease.Despite the association among disease along with switches in greater ratios is just a posit ,similar switches in Bifidobacterium along with Bacteroides have been seen in children having a chance for T1D generation.As both genera have the ability of metabolizing HMOs,this inverse association is believed to originate from inter particular competition regarding the same source ]112].Intriguingly these switches get parallel in contrast amongst Breast fed (greater Bifidobacterium) as well as formula fed(greater Bacteroides)infants.It is not astonishing ,an inverse association among Bifidobacterium colonization as well as T1Ddiseasegeneration has been seen in a lot of cross sectional along with longitudinal human studies[102,112].Furthermore Meija-Leon etal. recognized an escalated population of Bacteroides has been illustrated in recently diagnosed T1D[113].Akin to that a greater T1D incidence in Finnish as well as Estonia study population has been seen along with a greater prevalence of Bacteroides in at risk children in contrast to children at risk in adjacent Russia[61].
In the other DABIMMUNE study detailed earlier ,authors demonstrated that Bacteroides spp,like B. dorei, inhibited immune stimulation as well as inflammatory cytokine responses to E.Coli that lead to repressed innate immune signalling as well as reduced endotoxin tolerance [112].This immune repression gets manipulated by high amounts of Bacteroides-obtained LPS which are structurally as well as functionally separate from those generated by E.Coli ,the dominant kinds of LPS existing in Russian infants [112].This immune repression gets mediated Furthermore in vitro experiments demonstrate that LPS generated by different taxa can inhibit or stimulate toll like receptors(TLRs),TLR4, NFκB activation as well as endotoxin tolerance[161-67]. Particularly, when NOD mice got injected with an immunogenic LPS generated by E.Coli , endotoxin tolerance was evoked In addition to reduction in T1D incidence[112]. LPS from B. dorei, did not confer similar protection against T1D Initiation[112].These outcomes validate earlier NOD mouse studies where LPS was observed to have a direct effect on T1D propagation[112,115].The mode implicated in LPS manipulated immunity as well as its association with T1D generation are not clear totally ,but earlier work by Guiden etal.[116], as well as Wen etal.[55] have demonstrated the significance of toll like receptors(TLRs),TLR 3 as well as innate immune signal transduction Adaptor(MyD 88) in T1D Initiation in NOD mice ,that is 2 parts of the LPS/ TLR4 signal transduction pathway[55,112,116].
Together these outcomes from both in vivo along with human studies have found the significance of some Breast milk taxa in immune system generation along with manipulation.Certain genera,like Bifidobacterium have a significant part in the total health as well as generation of an infant , as well as have further been illustrated to have a particular, part in conferring protection to children at risk of T1D Initiation(fig1). Nevertheless, in current studies,even those infants who were only receiving Breast feeding during the initial 6mths of life had no Bifidobacterium spp, pointing that mothers are not getting colonized by these genera to the same degree as their predecessors.In a study from USA ,30% of the Breast feeding infants had no Bifidobacterium that could get detected , as well as for those infants getting Bifidobacterium colonization ,only 30%possessed Microbiota in which Bifidobacterium accounted for greater than 50% of the population [102,117].
3.5 Role of Dietary Factors
The total T1D incidence has escalated significantly in the last half of the twentieth century[117,118].Existing proof has suggested that Dietary factors aid in T1D Initiation.Certain factors have pointed to stimulate or escalate propagation of disease,whereas others confer protection against the generation of T1D associated autoantibodies along with total propagation of disease[59.117,119-125].Animal along with human studies have pointed that early life exposure to foreign food antigens,like gluten as well as bovine insulin can impact β cell autoimmunity[126]. Nevertheless,exactly the way along with the degree these Dietary factors influence disease results still has to be found
3.5a Gluten-Dependent Foods
A usual physiological property of T1D etiopathogenesis is an intestinal barrier that has become weak (alias leaky gut syndrome) that aids in escalated inflammation in T1D patients[34,127]. Gluten- is made up basically of monomeric gliadin along with polymeric glutenins that can further aid in escalating gut permeability as well as stimulate inflammation by cytokine liberation[128].Hence intestinal barrier function are key for suppression of inflammatory response . Gluten-Dependent diets are believed to aid in gut permeability via the manipulation of GM[129].Validating this taxa correlated with a Gluten-Dependent diets,like Akkermansia spp have been demonstrated to control human tight junction proteins,confer protection from pathogen invasion besides having a protective part against T1D initiation[130].
3.5c T1D initiation as well as Gluten-Dependent Foods
Till now the results on action of gluten-Dependent Foods have demonstrated lot of variability.A lot of studies have observed that putting patients on gluten -dependent Foods does not improve their antibody titres to a great extent . Nevertheless, same studies have further demonstrated that gluten-Dependent Foods can improve a person’s GTT[131].Few studies concentrated on patients with both celiac disease as well as T1D found a significant advantage with respect to improving health as well as DM regulation in those patients receiving gluten-dependent Foods[132]. Nevertheless, other studies have not observed akin actions[133].
Inspite of these inconsistencies ,1 factor has been commonly implicated as having a key part in generation of disease namely the time along with method of gluten administration.Human studies observed that early exposure (<3mths of age) to Gluten escalates the risk of islet auto immunity as well as this action gets ameliorated if Gluten is delivered when the child is still being given Breast feeding [43,134].Similar actions are observed in animal studies[135], as well as has been found to impact the initiation of T1D in a dose –based way[136].
The diet of the mother at the pregnancy time was evaluated in association with a child’s chances of T1D generation.On feeding the NOD mice mothers a Gluten-free Foods,the T1D incidence in their offspring got significantly decreased [137].In a study akin to this astonishing 7 times reduction in the T1D incidence in NOD mice pups occurred(62.5%-8.3%)[137]. Nevertheless, the outcomes from human studies have not been consistent.A current study by Antvorskov etal.,[138]observed that a cohort of Danish women,the risk of generation of T1D was directly proportional to how much gluten was consumed during pregnancy.,[138].Women having the maximum intake of Gluten(>20g/day) possessed double the chances of T1D initiation in their children. As compared to that,2 previous studies observed no robust chances among mothers as well as T1D incidence in the child[139].
Getting exposed in early life to diabetic Dietary factors possesses main impact on T1D initiation.Inspite of that deletion of Gluten from diet of older children having a diagnosis of T1D has been illustrated to enhance diabetic metrices that are propagation of remission periods along with decreased HBA1c[140].A study akin to that documented better glucose tolerance as well as insulin sensitivities ,but no advantageous action in relation to better titres of islet auto antibodies in older children put on Gluten-free foods for6mths(median age=16yrold)[131].
In total these research works have given key knowledge on the part gluten plays in T1D initiation. Nevertheless, human longitudinal studies are required to derive an association with diet, its impact on the Gut Microbiome , as well as how this influences T1D initiation as well as propagation.1 such study is the German longitudinal cohort study, BABYDIET study.
Babydiet Study
BABYDIET represents a substudy of the bigger BABYDIAB study that got started to find if gluten G intake in early life has a part in T1D initiation.This study had been following 22 children showing auto immunity as well as 22 matched control for slightly greater than 3yrs .They had stool samples procured mthly among ages 3mth-36mths along with 6mths interval following that .All the participants had a minimal of one first degree relative having overt T1D.In toto no significant variations were seen among cases as well as controls for the Microbial diversity (namely rich or evenness) along with composition once results had been corrected a lot of comparisons[141]. Nevertheless, on contrasting the total Microbiota network (Eigenvector Centrality (EC), Node Degree] of children showing autoimmunity vis a vis autoantibody negative children, significant differences were observed . Autoantibody positive children illustrated significantly separate centrality evenness in contrast to healthy controls at 6mth along with 2yrs of age .Further greater network nodes having intermediate connectivity in the autoantibody positive children cohort. Moreover, particular genera had differences in cases along with control in an age-based method.Like at 6mth of age ,the bacterial genera Enterococcus ,Prevotella , as well as Corynebacterium displayed greater EC at 2yrs of age in autoantibody positive children. As compared to that genera which documented greater EC at 2yrs of age in autoantibody positive children had Barnsiella as well as Candidatus Nardonella ,while Staphylococcus as well as Nocardioides possessed greater EC in the autoantibody negative children.It is significant to notice that these findings were at the genera level secondary to 16S sequencing.
3.5d Cows Milk Along with Its Proteins
Both animal model studies along with human ones have observed correlation among T1D incidence along with the intake of Cows Milk at both weaning time as well as later in generation.Retrospective meta-analysis on national dietary intake records have got utilized to find if national along with global standards of Cows Milk administration associates with regional T1D incidence rates[126,142,143].Various Evaluation conducted by Montoni etal.,observed a positive correlation among T1D along with an areas consumption of animal –dependent energy as well as the areas total milk supply [217]. A meta-analysis that particularly contrasted a countries T1D incidence rate with its yearly Cows Milk protein intake also observed a positive association with a countries T1D incidence rate with its yearly Cows Milk protein intake [126,143].A negative correlation among breast feeding (at 3mths) as well as T1D chances was further seen [126].
Type 1 diabetes mellitus Prediction as well as Prevention( DIPP Study),a Finnish Study evaluated usual food intake in children possessing extensive β cell autoimmunity along with observed that the usual input of Cows Milk products appeared to be one of the rare factors that had direct correlation with β cell autoimmunity.This was not seen with other dairy products ,like sour milk products like cheese ,that points that the enhanced chances of β cell autoimmunity arrives particularly from Cows Milk correlated proteins [143].Various Studies have evaluated the immune response to these proteins as well as observed escalated risk of Cows Milk particular IgG as well as IgA antibodies at the early T1D initiation[145].Intriguingly ,Elliot etal.,[143]observed that the total proteins intake did not associate with Type 1 diabetes incidence as the particular intake of Cows Milk -obtained β-casein(A1 variant) did[143].These findings point that the escalated humoral response gets exhibited in against Cows Milk proteins in T1D patients,that makes them a potential aetiological factor resulting in the autoimmune event leading to T1D initiation[145].
Whereas proof agrees with a part for Cows Milk proteins in T1D initiation,other studies have not observed any such association[146].On the other hand,other studies have pointed that Cows Milk dependent formula has a protective part against T1D initiation[147].Human studies , evaluating the association among Cows Milk proteins ,GM composition as well as T1D have been obtained from 2 main longitudinal human studies.i) Trial to Reduce IDDM in the Genetically at Risk (TRIGR ) as well as ii) Finnish Dietary Intervention Trial for the Prevention of type 1 diabetes (FINDIA). Whereas these studies do not particularly look at the correlation among Gut Microbiome along with T1D incidence,a crosssectional study obtained from these studies was later carried out to evaluate these actions.
3.5di) Trial to Reduce IDDMin the Genetically at Risk (TRIGR)
In this TRIGR study,external dietary proteins got postponed till 6-8mths of age as well as the generation of autoantibodies(insulin, GAD,IA2 ,ZnT8) was screened till the age of 6yrs[119].The objective of this study was to if adding breast milk with greatly hydrolyzed milk formula had the capacity of inhibition of T1D initiation.Infants possessing verified HLA-correlated proneness(n=230) along with a minimum of 1 family member having T1D recruited in the study.These patients were delivered either casein hydrolyzate formula or conventional formula wherever breast milk was not present as well as were later followed for till the age of ten.During this study ,17 children generated at lease one Autoantibody in the casein hydrolyzate group(17%) in contrast to 33 children belonging to the control group(30%).8 children in the casein hydrolyzate group(8%) as well as ,17 (16%) in the control group generated 2 0r > autoantibodies.Insulin autoantibodies were the commonest seen first – autoantibodies with anti-islet autoantibodies remaining a close 2nd .In total this study observed weak , correlation among formula introduction along with with an escalated generation of T1D. A greater current update of this study got published in 2018 observed that weaning to hydrolyzed milk formula did not decrease the chances of generation of T1D with an escalated disease chances upto a median age of 11.5 yrs[148].
3.5dii) Finnish Dietary Intervention Trial for the Prevention of type 1 diabetes (FINDIA)
FINDIA represents a multisite double blind –clinical trial where newborn infants got randomized to get either bovine insulin free cow’s milk formula(CMF, CMF group),a whey dependent hydrolyzed formula(WHF group),or a whey dependent FINDIA formula (FINDIA group)from where bovine insulin had been deleted .Among 2002-2005, subjects got enrolled from 3 Paediatric hospitals in Finland.113 infants with HLA-correlated proneness to T1D were randomly allocated to get one of the three formulas .These outcomes from the FINDIA study [149]point that delivering bovine insulin-free formula at the time of 1st 6mths of life decreases a child’s chances of generating β-cell autoantibodies by the age of 3 .Once the Microbiota gets seroconverted subjects got contrasted with non seroconverted subjects,an escalated amount of Bacteroides, along with reduced amount of Bifidobacterium. Evaluation of intention to treat ,6.3% of children in the CMF group,4.9% in the WHF group, as well as 2.6% of children in the FINDIA formula group were positive for a minimum of 1 autoantibody by the age of 3.These scientists observed that in contrast to ordinary formula, weaning to an insulin free formula (TRIGR group)diminished to cumulative incidence of autoantibodies by age 3yr in children at genetic risk of T1D.
3.5diii) TRIGR-FINDIA Cross-sectional study
In a study by De Goffau etal.,[32]GM composition was evaluated along with contrasted with autoantibody positive(n=18),who tested positive for a minimum of 2 autoantibodies) along with matched autoantibody negative –negative (n=18), children.These candidates got enrolled from TRIGR, as well as –FINDIA. in contrast to autoantibody negative children, autoantibody positive children possessed relatively greater amount of Bacteroides(Bacteroides genus). Nevertheless, at the species level only little Bacteroides spp were correlated with auto immunity, along with a greater degree of Firmicutes, like Clostridium perfingens,both of which are agreed to escalate gut permeability.This Evaluation further points that low amounts of Bifidobacterium adolescentis along with Bifidobacterium pseudocatenalutum(<12% in combination) have a significant association with escalated amounts of β cell auto immunity (figure1) .
3.6Antibiotics
Antibiotics get commonly utilized for the regulation of bacterial antigens, possessing a wide variety of actions on the GM.Exposure to antibiotics has been observed to diminish the bacterial diversity significantly, Nevertheless,various studies have demonstrated that adult GM usually recovers to simulate Microbiota before treatment[150].However, A 4day treatment with a mixture of 3 Antibiotics(meropenem,gentamicin as well as a vancomycin)resulted in the deletion of 9 bacterial spin adult men 180days Subsequent to antibiotics use[150].In the same study it was seen that an initial flourish of pathobionts like Enterococcus faecalis as well as Fusobacterium nucleatum along with a reduction of Bifidobacterium[150].
Usually on average basis ,a child in the USA receives 3 Antibiotics courses prior to the age 10[151].Inspite of common delivery to infants as well as children ,their actions on GM along withhow it associates with human diseases,like T1D is not totally clear.A study of infants , Exposure to antibiotics was correlated with reduced Clostridiales as well as Ruminococcus in the initial 3-9mths of life , along with postponed maturation of the microbiota[152].Conversely ,in a large Norwegian Mother along with Daughter Cohort study did not observe any correlation with acetaminophen utilization along with T1D chances.In this same study utilization of antibiotics was not associated with greater T1D chances in both Mother along with Daughter in the 1st 6-9mths of life[153].Akin to that TEDDY observed that frequency as well as utilization of antibiotics in the initial 4yrs of life had no impact on the chances of generation of Autoimmunity for T1D or Coeliac disease[154].A case control study that was population-dependent as well as included all T1D children born from 1997 did not observe any association among utilization of antibiotics along with generation of T1D[155].In a population-dependent mother child cohort which had all children born from 1996-2000 in Finland,that got a diagnosis of T1D,an escalated risk of generation of T1D was observed in children born to mothers consuming phenoxymethyl penicillins or quinolone prior to pregnancy.They posited that utilization of antibiotics by Mother’s might reduce the shifting of healthy microflora to the baby.An escalated risk of T1D was also observed when the mother’s consumed macrolides prior to pregnancy as well as child received macrolides in contrast to mothers-child pairs when none of them received macrolides. A greater risk of antibiotics ,as the definition said ,namely 7 or greater buying of antibiotics was also observed to have an escalated risk of generation of T1D. Bifidobacterium as well as Lactobacillus spp get correlated with a healthy microflora,being Specifically sensitive to macrolides, quinolones as well as penicillins.This study posited that macrolides, along with quinolones might escalate the risk of T1D in children by avoiding the generation DNA along with the enzymes in β cells leading to β cells demise[156]. Another study that was case controlled utilized THIN,i.e ,a UK population medical record database ,which has total medical records of approximately 10 million patients,for evaluation of the action of antibiotics exposure on both type1 as well as type2 diabetes mellitus.The study observed that evaluation of single antibiotic was not correlated with greater diabetes chance. Nevertheless,receiving 2-5 courses of penicillins, cephalosporins, macrolides,or quinolones had a correlation with greater diabetes chance, as well as intake of over 5 courses of quinolones was correlated with greater diabetes chance. Getting exposed to greater than 5 courses of penicillins further was association with greater diabetes risk[157].
Various studies have got conducted on biobreeding diabetes prone (BB-DP) rats ,LEW1.WR1 rats along with NOD mice to find out the influence of antibiotics therapy on T1D incidence as well as Gut Microbiome composition.Brugman et al.[158], studied male along with female BB-DP rats on a conventional(CON)plant dependent or hydrolyzed casein(HC)in continuity got antibiotics therapy with broad spectrum antibiotics Bactrimel(sulfamethoxazole as well as trimethoprim) along with colistin sulfate. antibiotics therapy when on the CON diet was observed to decrease T1D incidence as well as postpone the initiation to 30days ,while antibiotics therapy when on the HC diet was observed to confer protection against initiation of T1D[158].Further BB-DP) rats who generated T1D later were illustrated To have escalated amounts of Bacteroides in contrast to controls as well as rats which did not have T1D generation[158].Akin to this ,in 2012,Hara et al., Evaluated LEW1.WR1 rats that were infected with kilham rat viruses (KRV) for induction of T1D, observed that virus stimulated T1D or repressed by treating with antibiotic sulfatrim .Therapy with sulfatrim was further observed to repress the virus stimulated anti-islet responses in the LEW1.WR1 rats through down modulation of immune system.Moreover , infection with KRV viruses resulted in Bifidobacterium along with Clostridium escalation[159].On the other hand ,Livanos et al., observed that pulses of therapeutic dosage of the macrolide antibiotic tylosin tartrate(pulsed therapeutic antibiotics ,PAT) exaggerated T1D as well as insulitis generation in male NOD mice.The study further illustrated that the group that received PAT had greater amounts of Akkermansia along withEnterococcus in contrast to control groups that had greater amounts of Bifidobacterium[160].Hansen et al.,[161] conducted a study in 2012 that evaluated the early life therapy with antibiotic vancomycin on NOD mice.They illustrated that mice receiving vancomycin from birth till day 28 possessed a significantly lesser T1D incidence as well as greater clusters of CD4+T cells generating pro inflammatory cytokines.On the other hand , NOD mice who got vancomycin from age 8wks till T1D initiation possessed greater T1D incidence, as well as greater insulitis score along with escalated glucose amounts,that pointed that early life treatment significantly influences the T1D propagation.Further vancomycin treatment also influenced the Microbiota composition since Akkermansia muciniphilia was dominant in the Gut microbiome of all groups receiving vancomycin]161].In a study akin to that ,the actions of antibiotic therapy on T1D propagation in the offspring of NOD mice getting antibiotic therapy during pregnancy further revealed the significance of antibiotic therapy in early life[162].These authors gave during pregnancy a neomycin,polymixin B as well as sptreptomycin ,ie 3 antibiotic s to the NOD mice along with illustratedthat offsprings of mice getting antibiotic therapy had significantly greater protection conferred in contrast to mice who got antibiotic therapy immediately following birth as well as mice that NOD mice getting antibiotic therapy at 3wks of age.This study highlights the significance of antibiotic therapy in early life or prenatal antibiotic therapy to counter T1D since the age of mice getting treatment possessed a negative association with protection from type1 diabetes. Moreover, the antibiotic therapy was observed to remove gram negative Proteobacteria as well as result in gram positive bacteria predominantly belonging to the Firmicutes family. As per the studies documented,it is not clear if antibiotic therapy constantly escalates or decreases the type1 diabetes risk in animals,but it is always observed to impact the microbiota composition as well as T1D incidence along with proof to point that early tackling is more efficacious.
4.Type1 Diabetes along with GM:peeping beyond the bacterial species
Once sequencing got introduced ,it changed the manner by which researchers asked queries regards to both environmental as well as human correlated microbiota ,thus has made them utilize big data in a manner that was never feasible earlier.With the advancements of sequencing methods , greater researchers are trying to utilze these technologies for Evaluation of correlation among the human Microbiome along with autoimmune diseases.At present , type1 diabetes research, researchers have Evaluated the variations existing in gut proteome,virome along with metabolome of T1D patients.Here the advances that have been conducted in the omics field is detailed.
4.1 Gut Proteome
16S along with shotgun sequences studies give us information as far as the Microbiota composition of the Gut Microbiome is concerned .Whereas Proteomic studies are required to get an insight regards to the proteins manufactured by the gut microbiota.Occasional studies till date have got carried out for Evaluation of intestinal Microbiota Proteome of Type1 Diabetes patients. Nevertheless, in a study Pinto etal.,[162], Evaluated the variations in the intestinal microbiota Proteome among children with Type1 Diabetes that had got established(n=3) in contrast to Proteome of healthy children ((n=3).In control samples , bacterial proteinsfrom Bifidobacterium adolescentis, Bifidobacterium longum subspp infantis,Ruminococcus,Collinsella aerofaciens,Coproconus comes as well as Clostridium spp were observed to be the maximum enriched,whereas proteinsthat initiate from Eubacterialis rectales,Faecalibacterium Prausnitzii, Bacteroides dorei along with Bacteroidesuniformis differed among control as well as case samples(figure1).More currently ,in one study[163]stool samples from a newly originated Type1 Diabetes patients,islet- autoantibody positive along with low risksubjects were contrasted.
MetaProteomic Evaluation got used for differentiation of stool samples into 3 divisions depending on the existence of human(host-originated) along with microbiota originated proteins.In case of patients from newly originated Type1 Diabetes ,a significant decrease in microbial-correlated host proteins ,which sustain the mucus barrier,adhesion of microvilli as well as exocrine pancreas .Hence Type1 Diabetes patients possessed a greater prevalence of intestinal inflammation along with reduced barrier function.An earlier study that got published in 2016 laid the platform for large scale metagenomics studies by illustrating on a smaller level ,the way particular gastrointestinal Microbial communities as well as host particular
phenotypic association are linked to initiation of human diseases[22].Though the part of exocrine pancreas In T1D initiation is not clarified they illustrated that exocrine pancreas enzymes like amylase,carboxypeptidaseCPA1 as well as CUZD1 are lesser in amount in Type1 Diabetes patients[22].These early outcome seem to be attractive along with giving marked understanding regarding how T1D MetaProteome varies in function in contrast to controls .More research in this field will give greater knowledge in the propagation of disease as well as avoidance methodology.
4.2 Gut Virome
Viruses have been believed as potential stimulators of T1D initiation along with propagation[165]. Nevertheless,most of the research has concentrated on the part of viral infections in this event as well as co conclusions have been constructed out of that data .More currently researchers have evaluated thepart of the Gut virome.This Gut virome Is markedly less explored since it associates both states of health along with disease , Nevertheless, the properties of intestinal virome from birth towards the generation of autoimmunity possesses the capacity to become a significant part in getting insight in the etiopathogenesis of T1D. Utilizing the longitudinal Type 1 diabetes mellitus Prediction as well as Prevention( DIPP)cohorts, from a Fnnish Study,stool Viromes got acquired from 19cases(who became autoantibody positive prior to the age of 2 as well as later generated T1D along with matched controls at 0,3 as well as 6mths prior to the initiation of islet autoimmunity[165]. Viruses comprised 2% of all the sequences that were isolated ,having a main dominance of bacteriophages that got isolated in 52/96 samples(54.2%).Astonishingly ,only 10.4 % of samples possessed 1 or greater human Viruses like parechVirus,boca Virus,annelo Virus,entero Virus as well as /or sapo Virus.Kramma etal.[165],further observed no correlation among the Gut virome as well as islet autoimmunity[166].
A study akin to this was carried out in 2017[167], Evaluated stool samplesfrom 11 childrenwho had generation of autoantibodies in correlation with T1D(of these 5 had T1D generation). in contrast to Kramma etals[166] .,study this one observed a lesser amount of Circoviridae –associated sequences (this group of viruses included circovirus) as well as lesser virome diversity in cases of T1D.More significantly the workers found that enough proof was there regarding with time ,the generational propagation of viromes was significantly separate among the children who had autoimmunity generation from those who did not.The dynamic alteration in Shannon diversity with time was separate among controls as well as T1D cases for Microviridae T1D as well as Podoviridae.The Random Forest evaluation of age –discriminative contigs further pointed that there was variation among ` case as well as controls [167].This study illustrated viromes alteration took place prior to seroconversion that points to probable causality.Utilizing virome capture sequencing techniques a more current study evaluated the gut virome of pregnant women with (n=35) along with without(n=26)TID right through the Environmental Determinants of Islet Autoimmunity longitudinal studies.Two viruses ,picorbina virus as well as tobamovirus,were found with greater frequency in pregnant women with T1D,as well as the enrichment of 77 viruses was separate among the 2 maternal groups that included 88 enterovirus B kinds[168].
In toto, these results point that variations in the gut virome of T1D cases as well as propagators. Nevertheless,one needs to use a little cautious in trying to decipher these observations as very occasional studies utilizing a markedly small sample size have been carried out on this topic.Like in other new evaluations,variations in techniques among studies might result in the differences seen in the conclusions.
4.3 Gut Metabolome
In vivo studies have illustrated the significance of GM in the generation of host metabolites [169,reviewed by us ref 6].Of the metabolites known to get manipulated SCFA, Specifically have been illustrated to have important part in many respects regarding homeostasisin general.On intake of dietary fibres,it gets digested followed by fermentation in colon by GM into short chain fatty acids( SCFAS)(like butyrate or butyric acid (BA),acetate(AC) as well as propionate [169].Diets that possess high fibres change GM composition by promotion of the generation of SCFA –generating spp.Moreover a 4 times escalation in SCFA has been seen in breast fed infants in contrast to formula fed ones[170].
Further Diets that possess high fibres also have been demonstrated to decrease systemic inflammation[168].A study conducted by Trompette etal.,[171] showed that mice receiving these diets high in fiber possessed greater amounts of SCFA along with got protected from allergic inflammation of the lung [171].In case of human beings a reduction in diets fibres has got directly correlated with a reduction in SCFA- butyrate [171].This butyrate is the particular SCFA possessing great significance.Of its lots of parts ,it enhances glucose along with energy homeostasis ,acts as an energy substrate for the colonic epithelium along with represses colonic inflammation as well as carcinogenesis.Moreober generating spp.Moreover butyrate has a necessary part in manipulating the immune responseby stimulation of differentiation of regulatory T(Treg)cells, SpecificallyFox3p+ Tregs as well as inhibition of inflammatory cytokines,like IFNƴ[27,169,173].Intriguingly on feeding NOD mice,a diet that has been fashioned to escalate acetate as well as fbutyrate or butyric acid (BA),amounts ,they had a reduction in T1D incidence in contrast to controls[174].
In 2 longitudinal studies like TRIGR along with –FINDIA , SCFA generating spp like Bifidobacterium Adolescentis,Roseburia faecis, as well as Faecalibacterium Prausnitzii had a negative association with the autoantibodies amounts observed[32].Hence children who had 3 or greater autoantibodies had the least amounts of SCFA generating spp.The TEDDYstudy validated this point finding a greater prevalence of Microbial genes correlated with fermentation as well as SCFA generation in healthy controls.Intriguingly ,the taxon correlated with these functions , were not persistent as per the geographical area pointing that the function instead of the taxon is conserved in healthy children[19].Overall,these outcomes point that spp having the ability for generation of butyrate have the ability to confer protection against autoantibodies generation in at –risk children, more particularly different Clostridium clusters having the ability of generation of butyrate from acetate , along with Bifidobacterium spp that can result in generation of butyrate through lactate metabolism(figure1).
Akin to the observations seen in animal models ,human studies have illustrated a metabolic switch in patients prior to the autoantibody generation,insulitis , as well as /or T1D.Sardinia possesses the 2nd biggest incidence just following Finland,thus is a good area for T1D studies.In a Sardinia-dependent study , Culeddu etal.,[175] observed that gut microbial products,like p-cresol sulphate along with phenylacetylglycine were implicated in the grouping of T1D patients . Utilizing results obtained via the Finland-dependent DIPP longitudinal study, serum metabolomic profiles of children propagating to T1D got contrasted to controls not possessing autoantibodies as well as /or had generated T1D.For the subjects who had propagated to T1D,decreased amounts of succinic acid as well as phosphatidyl choline (PC)were seen at birth,whereas decreased amounts of triglycerides as well as antioxidants ether phospholipids were visualized in serum samples .Escalated amounts of pro inflammatorylyso PC’s , decreased amounts of ketoleucine along with escalated glutamic acid were the changes observed months prior to the seroconversion to autoantibody positivity [177]. Intriguingly, Metabolic profiles became partially normal for T1D patients following seroconversion.This points that autoimmunity presents later following an earlier change that might have impairment in Metabolic function[177].
5. Conclusions
Thus lot of work has been carried out to get total insight into the etiopathogenesis of T1D,how it originates along with propagates..One thing is apparent ,that Type1 Diabetes is not simply secondary to a single etiology but implicates multiple factors.Moreover the techniques utilized for evaluation of this complicated disorder might have an impact on the results seen.One big variation stems from the conclusions derived from animal studies vis a vis human ones.Despite animal studies giving us a lot of deep knowledge regards to the particular mode working ,lot of variations are there inherent to rat/mouse models make one use these data with a pinch of salt as far as human health applications are in question.Like histological reports have illustrated definitive variations regards to both the physiology of pancreas in mouse models along with propagation regards to etiopathogenesisof T1D in the β-cell islets[178].Furthermore epidemiological results on T1D documented a little male preponderance of the incidence rates prior to the puberty, nevertheless,the incidence is 2 times greater in male in contrast to females amongst the ages of 15-39[179].In case of mice female NOD mice generate T1D at a more greater speed in contrast to the male ones.There was one study that revealed a lot of Bacteroides genus associations were sex-based .A particular e.g is the B.ovatis that was observed to have a positive association with autoantibody positive male children whereas the same spp had a negative association with femaleautoantibody positive persons[32].Hence future study into these apparently elusive variations is required to find if sex variations are actually secondary to inherent variations in the GM.It is significant to know that scientists have isolated approximately 500 separate treatments to avoid or correct T1D in NOD mice models ,where neither of these therapies got translated into an efficacious method for human T1D [180].The variations amongst NOD mice model as well as human results point that one has to consider other animal models for research in T1D as well as concentrate on human studies.
With the advancesin sequencing method newer avenues for evaluation of human microbiota have got available in biomedical research.The part of microbiota regards to a lot of chronic life implicating diseases can be studied including T1D.Conversely till now maximum approaches have been bacteria centred.Very little virome ,proteome as well as metabolomic studies are there.Further no studies exist as far as phageome[181] , fungal microbiome [182], as well as archaeome [183] as well as meta tanscriptome [184]. GM along with its products remain to be a dark matter ,with our insight still restricted.
The other restriction regarding, GM composition is that we don’t know regarding any nasal[185] ,skin[186],or vaginal microbiota studies [187] in this field of T1D .These represent other significant mucosal surfaces for microbiota -host Crosstalk as well as might participate in the autoimmune diseases like T1D.
It is significant to realize that no definite causal association isolated till now with human disease along with microbiota.Rather than huge screening studies,a parallel strategy might be to concentrate on posit depending on particularmodes.The significance of microbiota -host Crosstalk in shaping the GM composition,via both contact-based as well as chemically modulated modes[188]. Current work point that community function ,instead of particular taxonomic composition might have an intrinsic part in disease generation [25].Overall ,future work needs to look into particular community Crosstalk along with total function to yield better insight of the differing disease causations .Like it was recently posited that existent of microbes that carry an insulin –like peptide might stimulate a ,molecular simulationmode in T1D as well as found viral insulitis[189].Further they revealed the existence of these viruses in human fecal as well as plasma samples.
Further recently Kahalehili et al. posited that the immunoregulatory phytochemcial, indole-3-carbinol (I3C) which is existent in cruciferous vegetables, would control the propagation of T1D in nonobese diabetic (NOD) mice. During digestion, I3C is metabolized into ligands for the aryl hydrocarbon receptor (AhR), a transcription factor that when systemically activated prevents T1D. In NOD mice, an I3C-supplemented diet led to strong AhR activation in the small intestine but minimal systemic AhR activity. In the absence of this systemic response, the dietary intervention led to exacerbated insulitis. Consistent with the compartmentalization of AhR activation, dietary I3C did not change T helper cell differentiation in the spleen or pancreatic draining lymph nodes.Rather , dietary I3C escalateded the percentage of CD4+RORγt+Foxp3- (Th17 cells) in the lamina propria, intraepithelial layer, and Peyer’s patches of the small intestine. The immune modulation in the gut was accompanied by alterations to the intestinal microbiome, with changes in bacterial communities seen within one week of I3C supplementation. A transkingdom network was developed to anticipate host-microbe interactions that were influenced by dietary I3C. Within the phylum Firmicutes, several genera (Intestinimonas, Ruminiclostridium 9, and unclassified Lachnospiraceae) were negatively regulated by I3C. Using AhR knockout mice, we validated that Intestinimonas is negatively regulated by AhR. I3C-mediated microbial dysbiosis was linked to increases in CD25high Th17 cells. Collectively, these data demonstrate that site of AhR activation and subsequent interactions with the host microbiome are important considerations in developing AhR-targeted interventions for T1D[190].
Moreover Calabrese et al reviewed the role of beneficial effects of Mediterranean diet along with prebiotics in prevention Type 1 diabetes mellitus (T1DM T1D) represents a chronic autoimmune disease resulting from a complex interplay between genetic proneness along with environmental factors. Regarding the latter, gut microbiota has a key part in the pathogenesis of T1DM, by affecting intestinal permeability, molecular mimicry, and modulating innate and adaptive immune system, as described in several previous studies. The composition of the gut microbiota is largely influenced by diet. Some observational studies have shown that a low fiber intake is associated with the development of many inflammatory along with immune-modulateded diseases. In this regard , the Mediterranean diet (MD), which is based on high consumption of cereals (preferably as whole grains), legumes, nuts, vegetables, fruits, olive oil, and fish, could play a protective role. Many of the characteristic components of MD have functional characteristics with positive effects on health and well-being. Eating habits are the main significant determinants of the microbial multiplicity of the intestine and the food components influence both microbial populations and their metabolic activities from the early stages of life. Moreover, food metabolites influence the immune response. The intestine is considered the primary site where food metabolites mediate their effects, through epithelial integrity or mucosal immunity. The compromised epithelial integrity allows the translocation of bacteria and/or the diffusion of their products, such as food antigens and lipopolysaccharides, from the intestinal lumen to the tissues, which could enhance the stimulation of immune cells, contributing to the pathogenesis of autoimmune diseases, such as T1DM. The intake of a high amount of fiber and therefore of prebiotics with MD allows the microbiota to have a good microbial balance. Moreover, as more dietary fibers are ingested, a higher amount of short-chain fatty acids (SCFAs) is produced by anaerobic gut microbiota, promoting gut homeostasis, to which also contribute tryptophan metabolites and omega-3-fatty acids. Furthermore, the higher intake of polyunsaturated fatty acids and omega-3-fatty-acids contribute to a better metabolic control[191].
Again Verduci etal.emphasized on encouragement of long-term breast-feeding for at least the first 6 months of life and the prevention of early complementary foods along with gluten introduction (before 4 months of age) as well as delaying cow milk introduction beyond 12 months of life. These detrimental feeding habits create a gut microbiota dysbiotic state that can contribute to the onset of T1D in infancy. Further , they detailed on the probability of adding probiotics, prebiotics and post-biotics in the avoidance of T1D especially in those high risk for T1D[192].
Giampioli tried to highlight the significance of FUT2 nonsecretory phenotype in high risk children. Children with non-secretor phenotype and those affected by T1D share low amounts of bifidobacteria, along with lower of short-chain fatty acids amounts , intestinal phosphatase alkaline and a high incidence of inflammatory bowel diseases. In this regard, host-gut microbiota dyad may represent a relevant contributor to T1D development along with propagation in view of to its key part in shaping host immunity and proneness to autoimmune conditions. The FUT2 gene is implicated for the composition and functional properties of glycans in mucosal tissues and bodily secretions, including human milk. FUT2 polymorphisms may considerably impact the gut microbiota composition and host proneness to viral infections as well as chronic inflammatory disease.They described how probably crosstalk among mothers' phenotype, host FUT2 genetic background as well as gut microbiota composition impact on T1D onset. The study of FUT2-gut microbiota interaction may add a new piece on the puzzling T1D etiology and unveil innovative targets of intervention to contrast T1D generation as well as propagation. Dietary interventions, including the intake of α-(1, 2)-fucosyl oligosaccharides in formula milk and the use of specific prebiotics and probiotics, could be posited[193].
Further minimal knowledge exists in reference to the environmental factors that can be associated to these alleles. Recent proof pointed that, among those environmental factors, dysbiosis (imbalance) in the gut microbiota might participate in the pathogenesis of T1D, affecting the integrity of the gut along with resulting in systemic inflammation along with auto-destruction of the pancreatic β cells. Various studies have isolated alterations in the gut microbiome composition in humans and animal models contrasingT1D subjects with controls. Those changes depicted by a greater amounts of Bacteroides along with lesser amounts of the butyrate-generating bacteria like Clostridium clusters IV and XIVa.The modes by which the dysbiotic bacteria as well as their metabolites crosstalk with the genome and/or the epigenome of the host leading to destructive autoimmunity is still not clear. As T1Dis a multifactorial disease, understanding the interaction between different environmental factors like the gut microbiome, the genetic and the epigenetic determinants that are associated with the early appearance of autoantibodies can expand our knowledge about the disease pathogenesis.Hence for better understanding into the crosstalk among the gut microbiome, susceptibility genes,epigenetic factors, and the immune system in the pathogenesis of T1D Elhag etal detailed the mode. It was also shown that the presence of a specific bacterial species such assingle-SFB) or Bacillus cereus in the female NOD mice procreated under germ-free conditions escalates the expression of the signature genes in Th17 cells likeIl17a, Il17f, Il22, Il1r1, and Il23r which may delay the onset of T1D through regulating the auto-immune response leading to enhanced gut integrity [reviewed in ref no 194 ]. Moreover, comparative gut microbiota evaluation among the NOD mice with and without T1D revealed a significantly greater amounts of four taxa that are associated with antibiotic-mediated dysbiosis, including Enterococcus, Blautia, Enterobacteriaceae species, and A. mucinophila, these taxa were shown to be associated with an accelerated progression into T1D (Figure 2) [reviewed in ref no 194 ].
Legend for Figure 2
Courtesy ref no-194-Comparison between the opposite effects of probiotics and early-life use of antibiotics in NOD mice. (A) T1D protective effects of probiotic bacteria in NOD mice: Lactobacillus johnsonii N6 enhances the expression of INFγ and indolesamine 2,3-dioxygenase enzyme (IDO) which in turn increase the production of claudin-1 tight junction protein that maintains the integrity of the gut epithelium. Lactobacillus casei has probiotic properties, lowering the number of the splenic CD8+ cytotoxic T cells and promoting the expression of the anti-inflammatory cytokines (IL2, IL10) leading to increased immune tolerance in the gut. VSL3# probiotic has immune-modulatory functions, enhancing the expression of IL-33 cytokine that is necessary to maintain immune-tolerance in the gut and mesenchymal lymph nodes (MLN). (B) Role of antibiotic mediated dysbiosis in enhancing autoimmunity and pancreatic β-cells destruction in NOD mice: dysbiosis mediated by the use of antibiotics enhances the growth of pathogenic bacteria which promotes the inflammatory response via the TLR pathway which influences the gut permeability leading to metabolic endotoxemia. Endotoxemia stimulates the autoreactive T cells in the pancreatic lymph nodes leading to insulitis and β cell destruction.
Growing evidence indicates that these bacteria can modulate the gut inflammatory response,
either by increasing the gut permeability leading to metabolic endotoxemia orby enhancing the translocating of the LPS into the circulation which increases intestinalpermeability through a TLR-4 dependent activation of FAK-MyD88-IRAK4 signaling pathway,leading to the generation of insulitis and beta-cell destruction as shown in Figure2 [reviewed in ref no 194 ]. Moreever , peptidoglycan isolated from Firmicutes and Bacteroidetes species inAkita T1D mice were demonstrated to escalate the risk of retinopathy in human retinal endothelial cells [reviewed in ref no 194 ]. While the absence of the myeloid differentiation innate immune adaptor gene (MyD88) in the NOD mice protects against T1D, it was observed that this protection is mediated by the gut microbiome, through escalating the expression of the immuno-regulatory enzyme indoleamine 2,3-dioxygenase (IDO) in the pancreatic lymphnodes [reviewed in ref no 194 ]. On the other hand, raising the MyD88-negative NOD mice under germfree conditions quickly triggers the onset of T1D ,[reviewed in ref no 194 ]. Moreover, a recent study revealed that raising of germ-free MyD88 knockout mice with a group of bacterial probiotics or segmented filamentous bacteria provides either partial or complete protection against T1D, highlighting the variability in the protection mechanism that is utilized by different bacterial species [reviewed in ref no 194 ]. Progression into T1D in BDC2.5X TCR islet-specifictransgenic NOD mice raised under a specific-pathogen-free environment was shown to be associated with an impaired gut barrier leading to microbiota-dependent endotoxemia, this endotoxemia can activate the migration of the islet autoreactive T cells to the pancreatic tissue leading to the initiation of T1D [reviewed in ref no 194 ]. Additionally, the protective NOD mice like the Idd3/5 and C57BL/6 mice, (which carry T1D protective alleles) have a distinct gut microbiome composition when compared to wild type NOD mice, showing differences in the relative abundance of Lactobacillus, S24-7, and Ruminococcus ..Although there are major variations amongst human and animal models in T1D correlated gut microbiota, some commonalities emerge mainly in the mechanism by which different microbiomes can influence the controlling functions of the immune system, mainly through the role of LPS and viral proteins which are known to be associated with the initiation of T1D [reviewed in ref no 194].
Thus finally concluding ,still lot of work needs to be done with regards to correlation of susceptibility genes,environmental factors along with solving the mystery of gut microbiota in initiation of T1D.Yet ensuring breast feeding trying pre/probiotics and diet changes like MD ,or FUT2 / AhR activation targeting might aid in helping till further insight is gained.