Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 12 - Issue 1 - 2025
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Farkhanda Qaiser1*, Hugh O’Reilly1, Aileen McCabe1,2
1Tallaght University Hospital, Belgard Square North, Tallaght, Dublin, D24 TN3C, Ireland
2Trinity College Dublin, 2 College Green Dublin, D02 VR66, Ireland
*Corresponding Author: Farkhanda Qaiser, Tallaght University Hospital, Belgard Square North, Tallaght, Dublin, D24 TN3C, Ireland.
Received Date: January 17, 2024
Accepted Date: January 22, 2024
Published Date: January 25, 2024
Citation: Farkhanda Qaiser, Hugh O’Reilly, Aileen McCabe. (2024) “Ethylene Glycol Toxicity in The Emergency Department: A Diagnostic Challenge.”, Clinical Case Reports and Clinical Study, 11(1); DOI: 10.61148/2766-8614/JCCRCS/166
Copyright: © 2024 Farkhanda Qaiser. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Emergency clinicians should suspect ethylene glycol poisoning in cases with suspected toxin ingestion and apparently very high point of care lactate readings. These high lactate readings can be factitious. Point of care testing machines typically use lactate oxidase to determine blood lactate concentrations which can result in false high lactate readings.
We present the case of an adult male who presented to the emergency department with reduced level of consciousness, tachycardia and tachypnoea after suddenly collapsing at home. A collateral history from the patient’s partner suggested that he had ingested antifreeze. His point of care testing venous blood gas showed metabolic acidosis with a lactate level of 31 mmol/l. Ethylene glycol poisoning was suspected and the patient was administered fomepizole and had haemodialysis making a good recovery with same.
This case highlights that ethylene glycol poisoning should be considered in cases with suspected toxin ingestion and apparently factitious high lactate readings from point of care testing. In suspected ethylene glycol toxicity, appropriate antidote and supportive management should be administered empirically as soon as possible.
Introduction
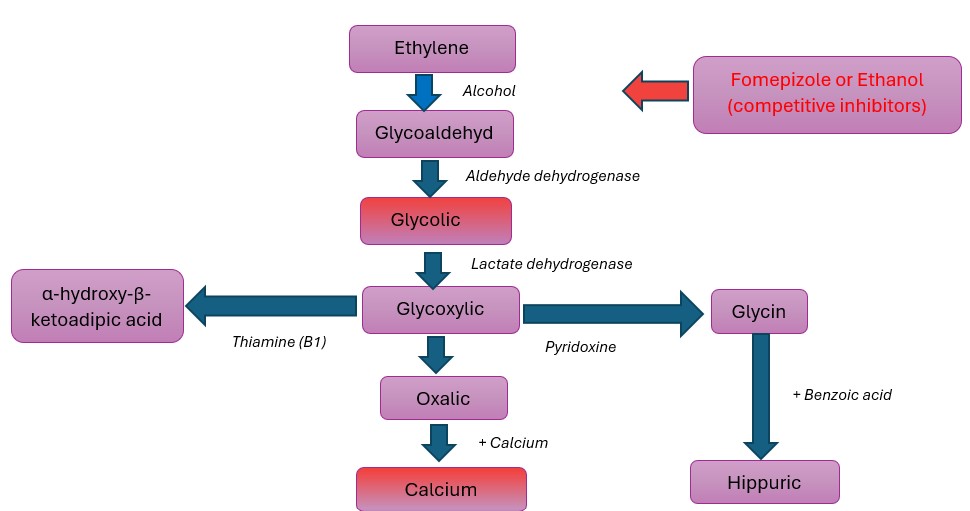
Ethylene glycol (HO-CH2-CH2-OH) is an odourless, colourless and a sweet tasting alcohol. It is present in antifreeze, brake fluids and many industrial solvents.1 It may be accidentally ingested, especially by children due to its sweet taste or intentionally ingested by adults as an inexpensive substitute for ethanol or in self-harm attempts.
This toxin is not itself injurious but its active metabolites glycolate and oxalate cause severe metabolic acidosis and acute tubular necrosis respectively.2 Moreover, glycolate is chemically similar to lactate and many cases have been reported3,4,5 in which glycolate was misinterpreted as lactate. Some point of care analysers use lactate oxidase to determine blood lactate concentrations and the incomplete specificity of this enzyme can cause cross reaction with glycolate.6 This can lead to factitious elevation of lactate levels in ethylene glycol poisoning.
Figure 1: Metabolism of Ethylene Glycol
Case
A 48 year old male presented to the emergency department by ambulance with a reduced level of consciousness after a collapse at home. A collateral history from the patient’s partner suggested that he had ingested antifreeze. His heart rate was 120 beats per minute, oxygen saturation at 99% on room air, blood pressure 150/110mmHg and respiratory rate 45 breaths per minute. His Glasgow Coma Scale (GCS) was low at 6/15 (E2, V1, M3). His pupils were bilaterally equal (size 3 mm) and reactive to light reflex. He was hypertonic. His abdomen was soft, non-tender and there was no guarding or rebound on examination.
The patient’s initial venous blood gas demonstrated a pH 6.98, pCO2 3.2 kPa, bicarbonate 8 mmol/L, lactate 31 mmol/L, sodium 152 mmol/L, potassium 5.9 mmol/L, chloride 124 mmol/L and blood glucose 7.3 mmol/L. The anion gap was calculated to be 25.9. The measured serum osmolality was 336 mOsm/kg and the calculated serum osmolality was 313 mOsm/kg, giving an osmolal gap of 23 mOsm/kg. His point of care urine toxicological screen was negative for benzodiazepines, opioids and tetrahydrocannabinol.
The collateral history of antifreeze ingestion and high anion gap metabolic acidosis with high osmolar gap raised suspicion of ethylene glycol poisoning. Tests for ethylene glycol levels were not available in our hospital and had to be sent to a specialised laboratory in the UK. The results were returned 4 days later.
Treatment was started empirically with intravenous fomepizole at a loading dose of 15 mg/kg. The patient was intubated due to unresponsiveness and airway maintenance and was transferred to intensive care unit for mechanical ventilation. He was treated with bicarbonate infusion for severe acidosis. Haemodialysis was started due to persistent severe metabolic acidosis.
The initial ethylene glycol level was reported 4 days later as 437 mg/l. The level dropped to 68mg/l on day two of admission following treatment with fomepizole and haemodialysis. The patient’s urinary oxalate level came back at 0.99 mmol/24 hours (reference range 0.04-0.5mmol/ 24 hours).
The patient improved with fomepizole and haemodialysis. He made a good recovery with same and was extubated and his haemodialysis was discontinued and he was discharged home well two weeks later.
Discussion
The diagnosis of ethylene glycol poisoning is challenging due to non-specific clinical signs and symptoms.7 It is a medical emergency that leads to deposition of oxalate8,9 crystals in the lungs, heart and kidney, producing organ dysfunction which may occur concomitantly10 or as three time-dependant clinical phases.11
The first phase (the neurological stage) occurs within 30 minutes to 12 hours after ingestion and includes ataxia, slurred speech, drowsiness, convulsions and coma. The second phase seen from 12 to 24 hours, comprises of cardiovascular effects such as hypertension or hypotension, arrhythmias and cardiac insufficiency. As these effects are due to the toxic metabolites so metabolic acidosis with raised anion gap, falsely elevated lactate, raised lactate gap and oxaluria may be detected. The last phase begins from day 2 and includes renal insufficiency with acute tubular necrosis. At this point, parent and toxic metabolites are generally not detectable. If untreated, death due to multi organ failure can occur 24 – 36 hours after ingestion.
A notable challenge is that direct measurement of ethylene glycol by high performance gas or liquid chromatography which is the definitive test is not routinely available in hospital laboratories.12 This assay requires specific expertise13; is costly and has increased turnaround times for the results because the samples are mostly sent for off-site testing. However it is a necessity to commence empiric treatment before a confirmed diagnosis as a treatment delay could worsen the mortality and morbidity.
The treatment14,15,16 comprises suppression of metabolism and removal of ethylene glycol and its metabolites from the body. Indications10 for treatment include serum ethylene glycol level above 20 mg per decilitre (3 mmol per litre); documented history of ingestion or likely strong suspicion thereof and an osmolal gap more than 10 mOsm per kilogram of water or unexplained metabolic acidosis. There is usually no need for gastric decontamination because gastrointestinal absorption of EG is rapid.
Table 1: Indications for treatment of ethylene glycol poisoning with an antidote (from Toxbase)
|
Recent history of ingestion of > 10 g (9.12 ml of 100%) ethylene glycol (EG) |
|
or |
|
Recent history (hours) of EG ingestion (any amount) and objective evidence of toxic alcohol exposure such as High anion gap metabolic acidosis or Osmolar gap > 10 mOsm/kg without another cause (e.g. ethanol intoxication) |
Treatment for ethylene glycol toxicity is either ethanol or fomepizole. Ethylene glycol is oxidized by the hepatic enzyme alcohol dehydrogenase.2 Prevention of its metabolism reduces levels of the toxic metabolites and is accomplished by the use of antidotes. Serum ethanol concentration10 of 100 mg per decilitre (22 mmol per litre) competitively inhibits this enzyme. The merits of ethanol include low cost and easy availability. However, ethanol administration can lead to unwanted effects like changes in mental status, hypoglycaemia and pancreatitis therefore frequent monitoring of serum concentrations and hospitalization in the intensive care unit is required.
Fomepizole is the preferred antidote. It is also a competitive inhibitor of alcohol dehydrogenase and stops formation of the toxic metabolites of ethylene glycol. It is superior to ethanol because of its nominal adverse effects; potency at low doses and no need of regular monitoring in the intensive care unit.17,18 The maximum effect of fomepizole is achieved when given early, before the formation of large quantities of metabolites. If the patient has adequate renal function and is non-acidotic then fomepizole might be the only 19, 20, 21 treatment required. The antidotal treatment is continued until plasma ethylene glycol level is less than 50 mg/L and acidosis and signs of systemic toxicity have resolved.
If the patient has severe metabolic acidosis, severe electrolyte imbalance, acute renal failure, deteriorating condition despite supportive care or ethylene glycol level above 50 mg per decilitre (8 mmol per litre), then haemodialysis22 should be employed immediately. The half-life of ethylene glycol is reduced to around 3 hours during haemodialysis instead of around 10 – 12 hours without this treatment.
Conclusion
Emergency physicians should suspect ethylene glycol poisoning in cases with suspected toxin ingestion and apparently factitious high lactate readings from point of care testing. In suspected cases of ethylene glycol toxicity, appropriate antidote and supportive management should be administered as soon as possible prior to waiting for definitive ethylene glycol levels which can have prolonged turnaround times.
Author contributions:
Author 1: Contributed to the study design, collected and analysed the data and drafted the manuscript.
Author 2: Assisted in data collection and provided critical feedback on the manuscript.
Author 3: Contributed to the study design and manuscript revisions and supervised the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None