Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Jared E. Mallalieu, D.O.
Laser Center of Maryland, 484-A Ritchie Highway, Severna Park, MD 21401
*Corresponding author: Jared E. Mallalieu, D.O. – Laser Center of Maryland, 484-A Ritchie Highway, Severna Park, MD 21401
Received: February 11, 2021
Accepted: February 17, 2021
Published: February 22, 2021
Citation: Jared E. Mallalieu, D.O. (2021) Melanoma in-situ associated with Melanotan II use. Clinical Case Reports and Clinical Study, 2(5) ; DOI: 10.61148/2766-8614/JCCRCS/028
Copyright: : © 2021 Jared E. Mallalieu, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Injectable synthetic melanotropic peptides may be used to enhance tanning, improve erectile function and treat female hypoactive sexual desire disorder (FSDD). The peptides, Melanotan I (MT 1) and Melanotan II (MT II) have been touted as sunless tanning agents, with the potential to decrease harmful effects of UV damage. Most recently, bremelanotide (PT141), an active metabolite of MTII, has been FDA approved for the treatment of FSDD. Numerous case reports of melanoma, eruptive nevi and atypical nevi appearing after initiation of melanotropic peptides therapy have been reported. To date, most of these cases have involved unregulated melanotan. This often called into question the dosing and purity of the peptide. We report a case of melanoma in-situ diagnosed four weeks after initiation of MTII use from a regulated compounding pharmacy. Clinicians must be aware of increasing melanotropic peptide use and screen patients accordingly given its unknown carcinogenesis risk.
Introduction:
Melanotans (Melanotan I and Melanotan II) are non-selective melanocortin receptor agonists. These potent synthetic peptides have binding affinity for melanocortin receptors MC1, MC3, MC4 and MC5. Melanotan I (MT 1) and Melanotan II (MT II) are synthetic peptide analogues possessing potency up to 1,000 times that of endogenously derived alpha-melanocyte stimulating hormone (α-MSH) [1]. MT 1, also known as afamelanotide, is a linear α-MSH analogue, while the newer MT II is a shorter cycler analogue. In many circles, MT I became known as the “Barbie drug,” due to its ability to stimulate melanocytes, thus resulting in a tan skinned appearance. Due to its mitogenic effect on melanocytes and its ability to increase levels of melanin, it has been proposed that melanotans may offer protection against the effects of UV damage. Additionally, studies have shown that the metabolite of MT II known as bremelanotide (PT-141), is effective in treating Female Sexual Desire Disorder. Despite some studies touting safety of the melanotan family of peptides, there are now increasing case reports of eruptive nevi, dysplastic nevi and melanoma in-situ associated with even short term melanotan peptide use [2- 9].
Case Presentation:
We report the case of a 66-year-old male who was diagnosed with melanoma in-situ after a four week period of self-injecting MT II obtained from Tailor Made Compounding (Nicholasville, KY). The injection consisted of only MT II without any MT 1. Injections were dosed according to Tailor Made protocol of 0.15ml injected subcutaneously once daily. During the course of injection, the patient exhibited skin darkening characteristic of MT II use. The patient reported no other side effects common with MT II use. Of note, a lesion on the right mandibular angle darkened substantially. The patient’s wife noticed the darkening and sent the patient for biopsy. The patient was well known to the practice and had routine skin checks. There was no history of tanning bed exposure, and the patient reported routine sun screen use. The patient is Fitzpatrick type 2 skin, with dark hair. The patient reported no history of abnormal skin biopsy or skin cancer. Furthermore, the patient reported no family history of skin cancer.
On examination, a 0.8 by 0.6 mm brown macule was noted at the right mandibular angle. Shave biopsy was performed and melanoma in-situ, Clark and Breslow level 1, was confirmed (fig 1). Diagnosis was made using routine hematoxylin and eosin stains. The patient underwent subsequent wide-local excision for treatment.
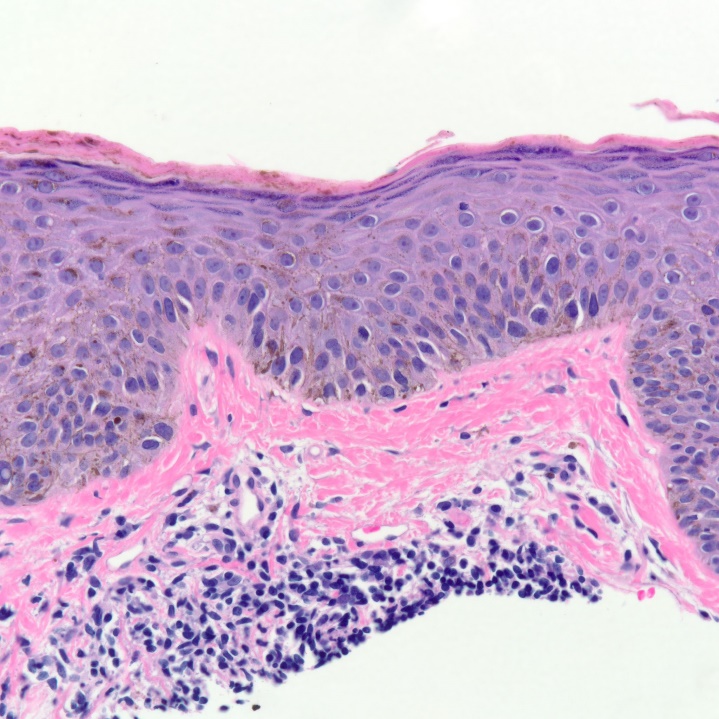
Figure 1: Melanoma in-situ at 40x showing melanocytes above the dermal-epidermal junction consistent with melanoma in-situ.
Discussion:
This observation brings attention to the potential risk and unknown effect of the melanotans in the pathogenesis of melanoma. Past reports have involved unlicensed and unregulated use of the various peptide forms. However, this case involved prescription MT II, produced under the structure of a compounding pharmacy. There is a growing body of case reports suggesting a temporal relationship between MT II injections and cutaneous melanoma and dysplastic nevi. Though no definitive cause and association has been identified, there is significant literature on the mechanism of α -MSH and the melanotan family of peptides and their effects on cellular behavior.
α -MSH acts in the skin through activation of the melanocortin 1 receptor (MC-1R), resulting in the cell shape change and increased dendricity [10]. In humans, MT I increases eumelanin production, associated with tanning and brown pigmentation, over pheomelanin, associated with red hair and burning [11]. This observation led some to believe MT 1 may confer UV protection [12]. However, research has shown that MC1-R gene variants increase risk for cutaneous melanoma. This increased risk is largely independent from skin type and hair color [13, 14]. In such individuals, increased activity by MT I or II could potentially induce melanoma.
The role of α -MSH as a potent anti-inflammatory agent is well known. Research has supported a possible relationship whereby α -MSH decreases Tumor Necrosis Factor-alpha (TNF-α). Substantial research on this relationship exists in the neuroscience field, where it has been shown that α -MSH can decrease TNF- α following exposure to inflammatory agents and cerebral ischemia. In this case the reduction of TNF- α could reduce tissue damage and improve recovery [15, 16]. Research has also shown that α-MSH can inhibit the release of TNF-α when exposed to endotoxin, as well as decrease reactivity of exposed monocytes [17,18]. The potent anti-inflammatory actions of α -MSH may be a double edged sword in the case of melanoma. TNF-α has documented anti-tumor activity in human cancer. Specifically in the case of melanoma, TNF-α has been used in isolated limb perfusion as part of chemotherapy strategies to treat advanced stage melanoma [19]. Furthermore, melanoma has been reported to develop in patients using TNF-α antagonists for Crohn’s disease and psoriatic arthritis [20,21]. Taken together with the fact that α-MSH reduces the ability of immune system to interact with melanoma cells, α-MSH may help melanoma cells evade detection [22].
The effect of α-MSH on pre-existing melanoma is somewhat unclear. Peroxisome proliferator-activated receptor –gamma (PPARγ) agonists have been shown to exert anticancer effects in several tumors, including some studies on melanoma [23]. α -MSH has been shown to upregulate PPARγ, potentially decreasing melanoma cell proliferation [24]. One study has also shown TNF-α to increase melanoma cell migration. This migration was inhibited by α -MSH, however, it only occurred in cell lines with intact MCR-1 [25]. Another study looking at melanotropic peptide on melanoma cell growth in mice found that the analogue did not increase primary tumor growth, or lung metastases in vivo using a murine model [26]. Other studies, however, have shown that melanotropins and α-MSH do not inhibit melanocyte growth, and in fact may stimulate growth of melanoma cell populations of the Cloudman S91 line in vitro [27, 28]. α-MSH does appear to decrease T-cell interaction with melanoma cells, thus helping melanoma to evade immune system detection in the first place [29]. Additionally, studies involving prognostic markers in patients undergoing immunotherapy have noted that weak expression of α-MSH, along with other immunosuppressive cytokines, is associated with longer survival [30].
In our case, along with some others, the short duration of MT II use calls into question whether MT is responsible for carcinogenesis. In theory the melanoma could have been present before the use of MT, and darkening of the lesion could have simply drawn attention to it. In this case, the use of MT could be used to key in clinicians to early melanoma years before their clinical features would otherwise dictate biopsy. Indeed, radiolabeling of various α -MSH analogs have been studied as a vehicle to help identify melanoma [31]. If the potential of carcinogenesis can be ruled out, MT could find a role in helping clinicians readily identify, localize and even treat melanomas.
Conclusion:
Though no study has definitively shown MT I or MTII to cause melanoma, the question of carcinogenesis remains answered. Numerous case reports have shown even short duration of use can induce changes in longstanding lesions. Melanoma carcinogenesis is a multifactorial, with many genetic implications. Furthermore, understanding of α -MSH and its peptide analogs are not complete. Given the action of α -MSH on melanocytes there is a real possibility MT I and MT II can help melanoma cells evade detection. Furthermore, it would appear family history and patient behavior play critical roles in development of dysplastic nevi, and that MT I and II may increase the likelihood of developing dysplastic and neoplastic changes in the face of MCR abnormalities. As the use of this peptide becomes more popular, health care providers must be aware of the potential risk of cutaneous melanoma arising in patients using melanocortin peptides in order to counsel and screen patients accordingly.
Conflict of Interest: None