Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Francesco Di Spigno MD1*, Alberto Catania MD2, Cristina Leoni MD2, GezaHalasz MD1and Mauro Codeluppi MD2
1Cardiology Department Guglielmo Da Saliceto Hospital; Piacenza, Italy
2Infectious Diseases Department Guglielmo Da Saliceto Hospital; Piacenza, Italy
*Corresponding Author: Francesco Di Spigno, Cardiology Department Guglielmo Da Saliceto Hospital; Piacenza, Italy.
Received: November 05, 2022
Accepted: November 26, 2022
Published: January 24, 2023
Citation: Francesco Di Spigno, Alberto Catania, Cristina Leoni, GezaHalasz and Mauro Codeluppi (2023) “FUSOBACTERIUM NECROPHORUM, NOT ONLY LEMIERRE’S SYNDROME”, Clinical Case Reports and Clinical Study, 1(8); DOI: http;//doi.org/11.2022/1.153.
Copyright: © 2023 Francesco Di Spigno. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
A 28-years old Salvadoran woman without any cardiovascular diseases was admitted to ER and hospitalized in Cardiology department for chest pain ; subsequently she manifested pharyngodynia, fever and cervical discomfort; the blood-cultures indicated growth of Fusobacterium necrophorum a Gram negative anaerobic rod usually implicated in a post-anginal sepsis associated with the frequent possibility of internal jugular thrombosis known as Lemierre’ syndrome.This clinical case whose evidence of pericardial flogistic involvement, a real rare complication of this kind of septic status,in the absence of jugular’s thrombus detected, could allow for discussion about a clinical-diagnostical problem in a really rare condition like this.
Introduction
Fusobacterium necrophorum is a gram negative anaerobic bacterium responsable for Lemierre’s syndrome, a human postanginal sepsis caused by the bacterium; thrombotic involvement of internal jugular vein is frequent and has been detected in 26 to 45% of cases1.
The first human infections of Fusobacterium necrophorum, usually causing zoonosis as reported by Loeffler, Flugge and Bang under other names, were described in 1891 by Schmorl who observed men that had beenworking on rabbits with necrobacillosis developing fingers’ abscesses with growth, from the purulent material extracted, of the characteristic filamentous gram-negative bacilli.
The first description of the isolation from a human of the bacterium now called Fusobacterium necrophorum subsp. funduliforme (initially named Bacillus funduliformis was the principal human pathogenic variety) was made by Jean Halle´ in 1898; Lemierre et al. did the first description of human systemic infection3 with F. necrophorum which was isolated from purulent synovial liquid in a child with chronic purulent otitis presenting with septic arthritis of the knee, cerebral abscess, and signs of overwhelming systemic infection.
Courmont and Cade in 19004 would have described Lemierre’s syndrome as a human postanginalsepticemic infection with F. necrophorum but the first recognized case of the sepsis resulting from inflammation of the throat associated with internal jugular venous thrombophlebitis was described by Long in 1912. This patient suffered from tonsillitis; after a few days of irregular septic fever the patient, blind in the left eye due to vitreous hemorrhage caused by cavernous sinus thrombosis (containing pus and bacteria) which had extended from the internal jugular vein through the inferior petrosal sinus. Lemierre5 credited Schottmüller with highlighting the importance of postanginal sepsis6 and Fränkel with recognizing the importance of the progression from thrombophlebitis of the tonsillar veins to internal jugular vein inflammatory thrombosis with subsequent septicemia also due to release of small pieces of infected thrombus.
Andre´ Lemierre (1875 to 1956) was a physician and professor of microbiology and infectious diseases at the Hospital Claude Bernard in Paris of brilliant clinical diagnostic abilities at
the bed-side and his role wasn’t to discover the organism now known as Fusobacterium necrophorum9, but he made it clear that, contrary to some previous reports , in which organisms other than anaerobes had been deemed to be the cause, postanginal septicemia was principally due to anaerobic gram-negative bacilli. However, Lemierre’s greatest contribution was his detailed clinical description of the postanginal septicemia associated with what we now know as F. necrophorumand the so clear diagnostic facilitation to physicians who have knowledge of the pathological condition.
Cardiac involvement is potentially dangerous and it is represented by endocarditis, myocarditis and pericarditis that could develop cardiac tamponade 8-12. We are presenting a case of pleuro-pericarditis of young women without any cardiovascular disease admitted to our Emergency Department-ER complaining of sharp, stabbing chest pain and fever.
Case Report
A 28-years old student women without any cardiovascular diseases was admitted to ER complaining of sharp, stabbing chest pain and fever (temperature of 38°C). Sitting up and leaning forward got ease up the pain, while lying down and breathing deep worsened it. Physical examination revealed the presence of pericardial rub and left cervical lymphadenopathy. Laboratory findings at hospitalization showed absence of leukocytosis with neutrophilia(5.76 x 103/ml, normal range 4-10 x 103/ml – Neutrophils 93.4%), increased level of C Reactive Protein (C reactive protein-CRP: 25.97 mg/dl, normal range 0-0.5 mg/dl), mild thrombocytopenia (initials PLT count 137000, normal range 150000 -450000/ml) and Prothrombin time prolongation (prothrombin time PT 58% normal range 70-120% /prothrombin time-international normalized ratio PT-INR 1.47)9. Repeated nasal SARS-CoV2 molecular swabs were negative as well as EBV sierology. On Electrocardiogram (ECG) there was sinus tachycardia. Echocardiography revealed normal left ventricle function, no valvulopathies and hyperechogenicity of pericardium, without pericardial effusion (Fig. 1). Chest X-ray was unremarkable. Otorhinolaryngology examination showed diffuse pharynx hyperemia with tonsillar hypertrophy (II degree, symmetric) without purulent exudate, swelling of cervical lymph nodes. Blood cultures resulted positive for Fusobacterium necrophorum and Angio-CT of the neck ruled out the presence of thrombosis of internal jugular vein underlying enlargement of left tonsil (diameter 23 x 20 mm) with disomogeneus enhancement (Fig. 2). The patient was treated with injecting amoxicillin-clavulanic acid, methronidazole and ibuprophene. Cardiac Magnetic Resonance (CMR) performed after few days revealed the presence of pericardial effusion at basal segments of anterior and antero-lateral walls in cine sequences, T2-weighted spin-echo negative and late gadolinium enhancement-LGE of pericardium in the same portions (Fig.3). Collaterally CMR showed mild left pleural effusion (Fig. 4). The hospitalization was unremarkable and the patient was discharged after two weeks of intravenous antibiotic therapy. At hospital discharge we have to underline the following restored hematological-biochemical values: RCP was 4.66 mg/dl and PLT 216000/ml.

Figure 1: T-T Echocardiogram showing iperechogenicity of infero-lateral wall.

Figure 2: angio-CT of the neck negative for signs of thrombosis
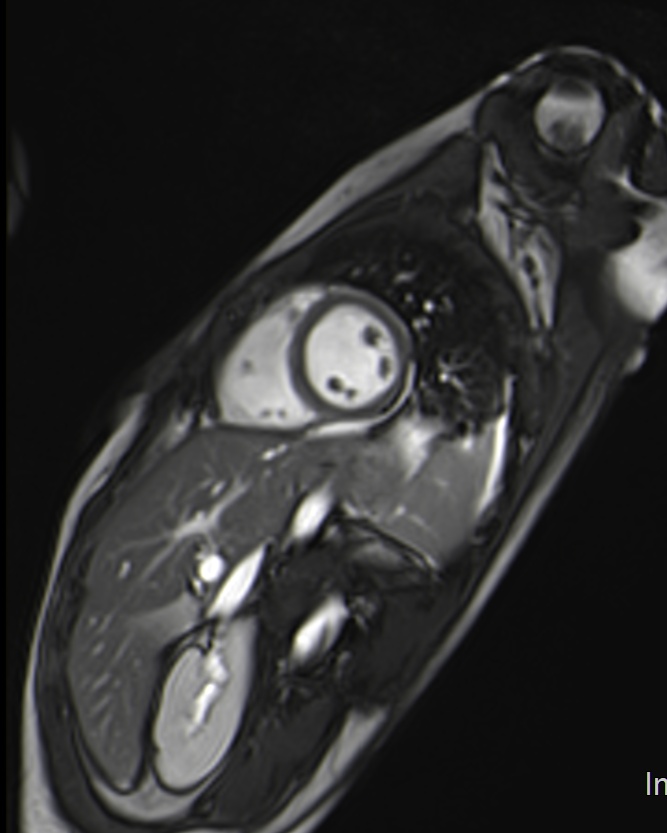
Figure 3: cine-RM sequences showing pericardial circumferential effusion

Figure 4: LGE sequences positive in pericardial layer
Discussion
Fusobacterium Necrophorum is a rod-shapedgram negative bacterium, obligate anaerobe, common inhabitant of alimentary tract within human and animals. It is responsible for Lemierre’s syndrome1, an infectious thrombophlebitis of internal jugular vein, due to development of a peritonsillar abscess as consequence of throat infection inducing sepsis with abscessual spread localizations (infarcts in the lungs with associated pleurisy or empyema but also liver and portal vein in cancer or after abdominal surgery); as we have seen, Lemierre and collegues first described the progression of tonsillitis towards internal jugular vein thrombophlebitis with subsequent septicemia.
A potential dangerous complication is also pulmonary thromboembolism. Cardiac involvement is quite rare and it is represented by endocarditis (six of the seven cases described in literature occurred before 1853), myocarditis and pericarditis. In the antibiotic era endocarditis is uncommon but require valve replacement. To date myocarditis was reported as mycroabscesses found in the myocardium at autopsy. Pericarditis and pericardial effusion were consequences of Fusobacterium Necrophorum bacteremia and they could even develop cardiac tamponade,with high mortality (30-75%),especially with purulent pericarditis8.
To date pericardial involvement isa rare complication. Kachman described a 31- year-old male with Lemierre's syndrome presenting with pleuro-pericarditis9, and Yuan et al. reported a case of a 26-year-old male with mediastinal abscess and purulent pericarditis10. McLean and Tyler had published a case of a 23-year- old primigravida with Lemierre's syndrome who had developed cardiac tamponade secondary to mediastinitis and anticoagulation treatment. In another report a 10-month-old child had Lemierre's syndrome, caused by rare staphylococcal infection, with hemorrhagic pericarditis while receiving anticoagulation 11, and Root et al. described another 10-month-old child with Lemierre's syndrome who developed purulent pericarditis and tamponade while on treatment12.
Echocardiography is the initial method of choice for evaluating most pericardial diseases. The main limitations are the dependence on a good acoustic window and the inability to investigate the entire pericardium9. In this sense the application of contrast echocardiography could be useful in the detection of pericardial effusion especially in the acute setting of myocardial infarction-MI with pseudo-aneurysm and free-wall rupture.
CMR and computed tomography-CT are considered the preferred imaging modality to investigate morphologically the pericardium and pericardial space.13
Cardiac CT could properly visualize coronary arteries and it provides high-resolution imaging of all cardiac structures, including the pericardium. The administration of intravenouscontrast with enhancement of pericardium can be observed in case of suspected pericarditis or tumor infiltration10. However, the CT attenuation of pericardium is similar to that of the myocardium. Hence, the pericardium can only be clearly visualized, where it is surrounded by fat and not immediately adjacent to the myocardium.
Black blood T1-weighted spin echo CMR is the best sequences to investigate the heart, pericardium and mediastinum11. T2-weighed spin-echo CMR with short-tau inversion recovery (STIR) is useful to highlight myocardial edema and pericardial fluid and/or edema of the pericardial layers in inflammatory pericarditis14. The application of paramagnetic contrast agents can better characterize pericardial masses, inflammatory pericarditis, concomitant involvement of myocardium (e.g. myocarditis) and may be useful to better differentiate between inflammatory and constrictive forms of pericarditis15.
Conclusion
We are describing a case of septic infection starting by upper airways inflammation caused by Fusobacterium necrophorum, with evident limphoadenopathy more significant on left side. The anaerobic bacteria is becoming an emerging pathogen in last years.7
Among patients affected by this rare anaerobic bacteria with tonsillitis or throat inflammation, and associated septic diffusion,it’s important for the clinician, beyond investigation of eventual presence of usually unilateral internal jugular thrombophlebitis indicating Lemierre’s Syndrome, to early recognize the eventual presence of cardiac involvement that is a life threating complication. There is no time to lose and you have to start empirical treatment based on clinical signs. Finally the use of second level’s imaging (CMR, CT) is mandatory when transtoracic echocardiogram is inconclusive.
Declarations
Ethics approval and consent to participate:
Not applicable
Consent for Publication
All the patients involved in the current manuscript gave their consent for publication
Availability of Data and Materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Competing Interests
None
Funding
None
Authors' Contributions
FDS and AC collected the data and draft the manuscript. GH, CL, MC revised the final manuscript and contributed substantially to the study manuscript design. All authors read and approved the final manuscript."
Institutions where the work was performed: Guglielmo Da Saliceto Hospital, Piacenza, Italy
Disclaimer: None
Acknowledgements
None
Conflict Of Interest
None declared.