Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Isaac Alsallamin1*, Christian M. Jacobson1, Afnan Alsallamin1, Deema Chakhachiro1, Jehad Zeidalkilani1, Rami Musallam1, Faris Hammad1, Francisco J. Somoza-cano1, Anastasiia Weiland1, Abdul Rahman Al Armashi1, Ameed Bawwab1, Said Abueida2, Bayan Abualrob2
1Internal Medicine, St. Vincent's Medical Center, Cleveland, USA.
2Internal Medicine, Almakassed Hospital, Jerusalem, Palestine
*Corresponding author: Isaac Alsallamin, Internal Medicine, St Vincent Charity Medical Center, Cleveland, USA.
Received Date: January 27, 2022
Accepted Date: February 04, 2022
Published Date: February 08, 2022
Citation: Isaac Alsallamin, Christian M. Jacobson, Afnan Alsallamin, Ameed Bawwab, Faris Hammad, Said Abueida, Bayan Abualrob (2022) “An Unusual Presentation of Autoimmune Encephalitis Presenting with Space Occupying Lesion.” Clinical Case Reports and Clinical Study, 7(2); DOI: http;//doi.org/01.2022/1.128.
Copyright: © 2022 Isaac Alsallamin. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Acute disseminated encephalomyelitis (ADEM) is an acute presentation. Presentation varies from abnormal sensation, behavioral changes, weakness, seizure to coma. The most common imaging finding is the presence of widely scattered foci of perivenular inflammation and demyelination that can involve both white matter and gray matter structures.
A 17 years old male who initially presented to the hospital with right leg weakness, which by the end of that day progressed to include the entire right side of the body. Computed tomography scan of the brain was performed and demonstrated a hypodense area of the left upper parietal centrum semiovale. A subsequent magnetic resonance imaging scan of the brain showed a large space-occupying lesion at the left parietal area.
The patient was maintained on pulse steroids and received seven sessions of plasmapheresis and five doses of intravenous immunoglobulin. Full recovery was achieved after a period of 6 months.
ADEM is considered a diagnosis of exclusion. Early imaging is crucial for the diagnosis and management of ADEM. Patients who fail to respond within a few days may benefit from a course of plasma exchange or intravenous immunoglobulin.
Introduction
Acute disseminated encephalomyelitis (ADEM) is an acute onset single-monophasic neuropsychiatric presentation [1-3]. ADEM is more common in pediatrics than adults [4-7], but less likely in young and middle-aged adults [1,2,8,9]. Presentations range from abnormal sensation, behavioral changes, weakness, seizure to coma [2-3]. Most of the cases reported after a viral infection, very rare cases after vaccination, recently some cases and case series reported after COVID infection [10-13]. The most common imaging finding is the presence of widely scattered foci of perivenular inflammation and demyelination that can involve both white matter and gray matter structures on the opposite side to MS [1,4,9,14,15]. Imagining unlikely to show space occupying lesions, and it's very unlikely to show rapid growing structures as reported in this case [1,15].
Case Presentation
The patient is a 17 years old male who initially presented to the hospital with right leg weakness, which by the end of that day progressed to include the entire right side. This was later accompanied by fever of 38.4 °C and focal convulsions on the right side lasting for 5 minutes. Following this, his speech became noticeably slurred, there was a delay in mentation, and cognitive impairment. Computed tomography scan of the brain was performed and demonstrated a hypodense area of the left upper parietal centrum semiovale [Fig 1]. A subsequent magnetic resonance imaging scan of the brain showed a large space-occupying lesion at the left parietal area [fig 2-7].
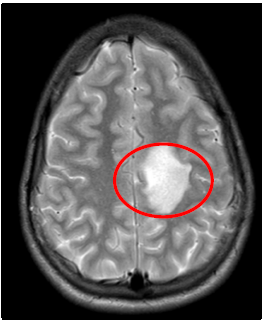
T2W: left parietal hyperintensity lesion
Fig1. Brain CT scan, Hypodense Area of the left upper parietal centrum semiovale.
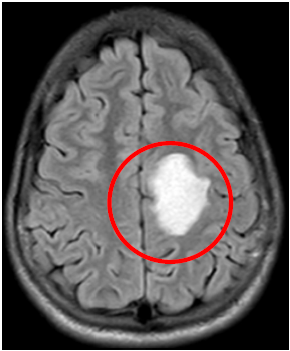
Axial; Flair, left parietal hyperintensity lesion.
Fig2. Brain MRI, T1W Sagittal view, Left Parietal hypodense lesion, space occupying lesion
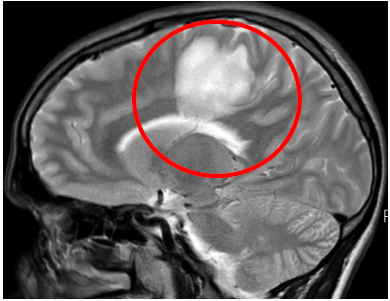
Sagital- T2W: left parietal hyperintensity lesion
Fig3. Brain MRI, T2W Sagittal view, Left Parietal hyperintensity lesion, space occupying lesion
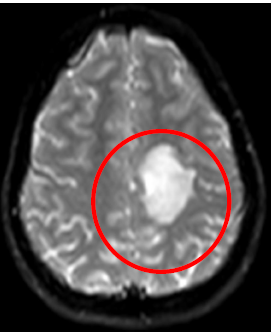
DWI: left parietal hyperintensity lesion
Fig4. Brain MRI, T2W Axial view, Left Parietal hyperintensity lesion, space occupying lesion, space occupying lesion, Mass effect, no signs of herniation.

PAC: left parietal hyperintensity lesion
PCA Sinus sense
Fig5. Brain MRI, DWI Axial view, Left Parietal hyperintensity lesion, space occupying lesion
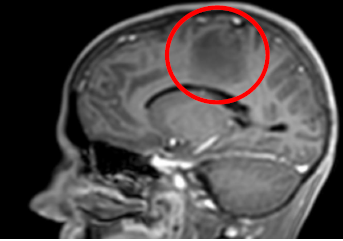
T1- Sagital: Left parietal hyperintensity lesion
Large space-occupying lesion at the left parietal area
Fig6. Brain MRI, FLair Axial view, Left Parietal hyperintensity lesion

CT Scan: Hypodense area of the left upper
parietal centrum semiovale
Fig7. Brain MRI, PAC sinus Sense
Following day, the patient suffered an episode of status epilepticus requiring intubation and sedation. He was treated with phenytoin, vancomycin, ceftriaxone, acyclovir, and solumedrol. An urgent repeat CT scan of the brain showed diffuse edema of the left hemisphere, mainly the centrum semiovale and periventricular region, with a mild mass effect on the left ventricle, without evidence of brain herniation. A lumbar puncture was performed; Analysis showed: WBC: 1200 cells/mm3 with 97% neutrophils and 3% lymphocytes; glucose: 71 mg/dL; and total protein: 56 mg/dL. Two cultures of CSF yielded no organisms. CMV and EBV serologies were negative for acute infection. 25-OH vitamin D level was 22 ng/mL. Anti-NMDA (N-methyl-D-aspartate (NMDA) and anti-GAD ( anti-glutamic acid decarboxylase) antibodies were undetected. After discontinuation of sedatives on day 6 an EEG showed no epileptic activity.
Subsequent care for possible demyelinating disease was delivered. The patient was maintained on pulse steroids and received seven sessions of plasmapheresis and five doses of intravenous immunoglobulin. He was successfully extubated three days after receiving this treatment. Recovery was gradual and required intensive physical and occupational therapy. Full recovery was achieved after a period of 6 months. The MRI brain at that time showed no evidence of demyelination or subclinical disease.
Discussion
ADEM as a demyelinating disease, presented with acute onset attack of neuropsychiatric symptoms, it's a single attack (monophasic), on imaging it shows involvement of both white and gray matter (scattered spots) [1-5].
At any time, recurrent episodes or development of chronic sequel an alternative diagnosis must be excluded, for example MS [8,9].
ADEM Diagnosis required extensive work up, including brain imaging and CSF analysis to exclude other pathology. ADEM is considered a diagnosis of exclusion [6-11].
Imaging findings are very important for early diagnosis of ADEM, but it may also be misinterpreted as mass effect on a CT scan, or standard MRI. ADEM diagnosis may require more detailed Imaging. Advanced MRI with MRA/DWI, flair, or other advanced sequence [11-15].
Management includes high-dose glucocorticoids. A course of plasma exchange or intravenous immunoglobulin may be required for resistant cases [1,3,6,7,14]. Multi-disciplinary approach to treat this disease and its complication management, and symptomatic management, required as in our case, mechanical ventilator required to protect airway, and respiratory support, anti-seizure medication for status epilepticus, antibiotic for aspiration pneumonia was given and treated accordingly [1,3,7,14].
Conclusions
Acute presentation of abnormal neuropsychiatric symptoms, rapid evolving, not clear underlying pathology required Brain imaging and CSF analysis. In our case a mass like occupying lesions seen on CT scan and MRI T1, our patient clinically rapidly deteriorated with a high suspicious for fast growing tumor, required proper work up, CSF analysis, EEG, and repeat brain imaging with full sequence MRI, help to diagnose ADEM, proper treatment initiated which saved our patient life, and prevented further brain damage or complications.
In this case we are trying to increase physicians' attention toward ADEM disease in adults. focus on acute presenting, not only typical brain MRI finding of multiple scattered foci, but it could be presented as a unilateral mass like occupying lesion, in the context of clinical picture.
Early detection helps to initiate proper management and to prevent severe complications or poor outcomes. Required high dose of steroids, and immunotherapy.
Our Recommendations for first episode of suspected ADEM disease especially in adults: surveillance and close follow up for recurrence which may change the diagnosis to MS later on.
Acknowledgement
Dr. Keyvan Ravakhah, Internal Medicine Program Director, for his help, advice, approval and reviewing this article before submission.