Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
E. Micelli1,2*, R. Fucci1*, L. Badolato1, R.Antonelli2, R. Picone1, P. Falcone1, C. Giachini1, F.Bertocci1, G. Cito3, P.Evangelisti1, F.Rizzello1, E. Coccia1
1Division of Obstetrics and Gynecology, Center for Assisted Reproductive Technology, Careggi University Hospital, Florence, Italy
2Division of Obstetrics and Gynecology, Santa Chiara University Hospital, Pisa, Italy
3Department of Urology, Careggi Hospital, University of Florence, Florence, Italy
*Corresponding author: E. Micelli, Division of Obstetrics and Gynecology, Santa Chiara University Hospital, Pisa, Italy
Received: December 15, 2021
Accepted: December 31, 2021
Published: January 10, 2022
Citation: E. Micelli, R. Fucci1, L. Badolato, R.Antonelli, R. Picone, P. Falcone, C. Giachini, F.Bertocci, G. Cito, P.Evangelisti, F.Rizzello, E. Coccia “Trichorionic Triamniotic Pregnancy After Single Blastocyst Transfer from A Double Donation of Gametes.” Clinical Case Reports and Clinical Study, 2(7); DOI: http;//doi.org/12.2022/1.122.
Copyright: © 2022 E. Micelli. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
,
Introduction:
The development of the zygote (16-18 hours post insemination) from the union between the oocyte and sperm, through different stages of cell division originates a morula with 16-18 cells ( 4th day post insemination) and then blastocysts 120-150 cells (5th day post insemination); the blastocyst has two distinct cellular components: the trophoblast from which extraembryonic tissues will originate and the inner cell mass from which embryonic tissues will develop [1].
Twin pregnancies have an incidence rate of 1-2%. The incidence of twin pregnancy is progressively increasing and the probability of twins newborn deliveries is estimated at around 1/90 pregnancies in the USA.
Generally, 70% of twins are dizygotic ones derived from two different fertilized eggs and 30% of them are monozygotic twins derived from a single fertilized egg [2].
Twin pregnancies can be divided into dizygotic pregnancy which are the results of two different oocytes inseminations and which are necessarily dichorionic diamniotic (DZ-DCDA)
On the other side monozygotic twin pregnancies derive from the more or less early subdivision of the product of the fertilization of an oocyte by a sperm, in particular:
• If the separation occurs early within the first 72 hours after fertilization, two embryos with the same genetic patrimony are obtained, each one with its chorion and its amnios, monozygotic dichorionic diamniotic (MZ-DCDA)
• If the separation occurs between the 4th and the 8th day after fertilization, when the differentiation of the trophoblast has already occurred but before the amniotic cavity has formed, a monochorionic diamniotic pregnancy is obtained (MZ-MCDA)
• If the separation occurs after the 8th day after fertilization, the chorion and the amnios have already formed and a monoamniotic monochorionic pregnancy is obtained (MZ-MCMA)
• If the separation occurs at an even later stage, a conjoined twins pregnancy is obtained [3-4]
In assisted reproduction (ART) after in vitro fertilization, the individual cells (blastomeres) of each embryo divide every 12-24 hours so that the embryo reaches the 8-cell stage within approximately 72 hours from the union between the gametes. [3]
The blastocyst stage is reached approximately by the 5th day and the implantation should take place by the 7th day. The transfer of the embryo into the uterus can take place in several stages: between 4-8 cells (cleavage stage) and blastocyst stage.
Therefore the division of the embryo into the blastocyst stage generally results in a MCDA or MCMA pregnancy. However recently DCDA twin pregnancies after single blastocyst transfer have been reported.
In this case report, we want to expose the circumstances of a completely exceptional event, triamniotic trichorionic triplet pregnancy that developed from a single blastocyst transfer in utero.
Case report:
The patient was a nulliparous 39-year-old woman with diagnosed premature ovarian insufficiency with no other comorbidities; her husband was a 43 healthy man with a history of severe oligoastenoteratozoospermia (OAT). They reported two previous failures of autologous gametes ART attempts. In the described cycle of ART the couple asked for the oocytes and semen donation program so they obtained a pool of six oocytes from a 30years old healthy woman and a semen donation from a healthy 24 years old man. A protocol of thawing (vitrified warmed ) of six donated oocytes was performed with the method described by Kuwayama et al. [5]. The woman underwent an endometrial preparation protocol with down-regulated trough a single depot-dose of a GnRH agonist (triptorelin) (Decapeptyl® 3.75; Ipsen Spa, Milan, Italy). After menses, the woman was administered oral estradiol valerate EV (Progynova®, Bayer, Milan): 2 mg/day for 5 days,4 mg/day for 4 days and 6 mg/day on day 11 until Embryo transfer (ET). Eleven days after initiating EV, patients underwent an endometrium evaluation by transvaginal ultrasound and serum estradiol (E2)/progesterone measurements.
A triple layer endometrium of at 9 mm was recorded and progesterone supplementation with 400 mg intravaginal capsules (Progeffik®/Prometrium®)every 12 hours was started the same day of egg warming. The six thawed oocytes were inseminated with ICSI with semen from the donor. Six oocytes were fertilized and a fresh blastocyst (grade 4AA) was transferred on the fifth day (Fig.1). Five blastocysts on day 5 (grade 2BB, 2BB, 4AA, 3BB, 4 AA [6] were vitrified on a cryotop.
ET was performed with the use of ultrasound guidance and an ET catheter (Guardia Access K-JETS-7019 or Guardia Access ET K-JETS-7019-ET; Cook) on day 5 at the blastocyst stage and one blastocyst was transferred into the uterine cavity.

Fig.1: Transferred blastocyst (day 5)
No ovulation in this patient and no intercourse between the couple during the treatment were recorded. The therapy (6 mg EV and 800 mg P) was continued until the 10th week of pregnancy.
Three gestational sacs and fetuses with heartbeats showing lambda sign were recognized at 6 weeks of gestation, so it was a triplet Trichorionic Triamniotic pregnancy (Fig. 2). At 10 weeks One gestational sac presented an embryo without heartbeat. Two days later the women referred to the emergency care after an hemorrhage. At ultrasound evaluation to signs of pregnancy were seen in uterine cavity. This case report has been written according to the CARE guidelines. The patient gave the consensus to the publication of data.
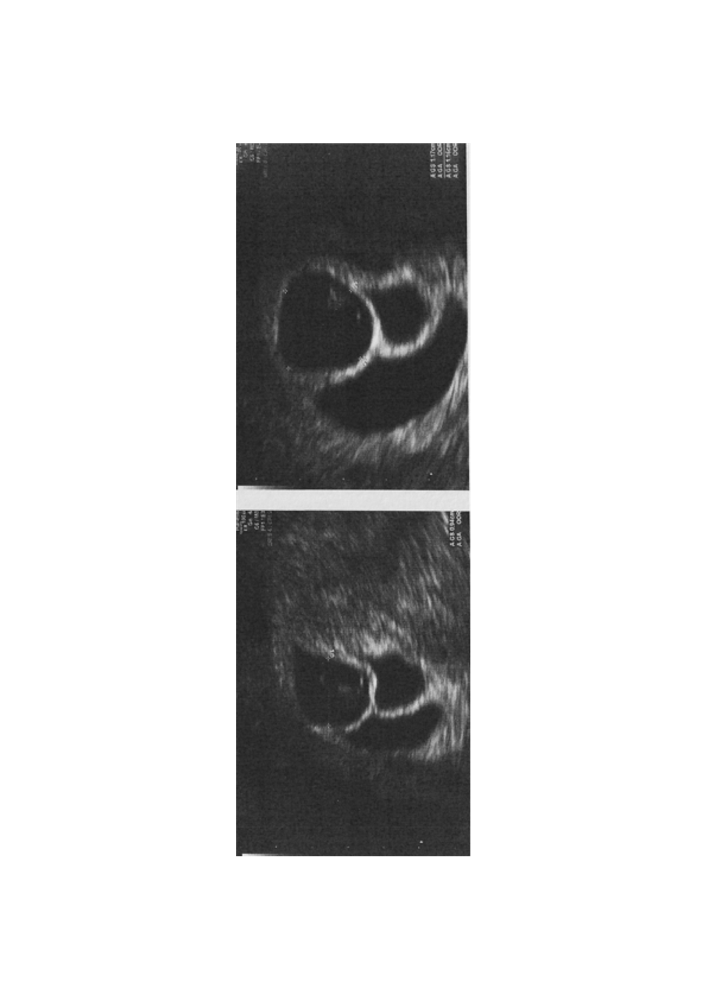 Fig.2: Ultrasound scan showing three gestational sac with lambda sign (trichorionic, triamniotic pregnancy)
Fig.2: Ultrasound scan showing three gestational sac with lambda sign (trichorionic, triamniotic pregnancy)
Discussion:
In 1955 Corner proposed a hypothesis of twins separation that constituted a dogma for numerous years. The Corner model hypothesizes four stages of embryological cleavage which located in specific temporal spaces of development: the 2-cell stage and before the compaction of the morula (days 0–3) generates DCDA twins, at the blastocyst stage the internal cell mass (day 4–8), evolved to MCDA twins, the bilaminar embryonic disc in the advanced stage of the blastocyst (days 9-12), creates MCMA twins and the primitive strip (> 13 days)originates Siamese twins [6]. This hypothesis quickly assumed the authority necessary to create an impeccable embryological model. However, as Corner himself assumed when he disclosed "unless the age of test tube children arrives this of human twinning must remain a plausible conjecture" [8-9] with the arrival of ART, a series of unexpected scenarios appeared.
The incidence of monozygotic pregnancies in ART is estimated between 1.2 and 8.9% and is usually a monochorionic pregnancy due to the presence of two inner cell mass within a trophectoderm [10-11]. A prospective study on twin pregnancies shows that in ART, only about 20% of all monozygotic pregnancies are DCDA [12]. However, the real number of DCDA monozygotic pregnancies is often underestimated as it is not possible to predict in how many cases of two embryos transfer, the twinning derives from the doubling of one of the two, with no implantation of the other transferred embryo. The observation of a DCDA twin pregnancy following a transfer of two embryos is, actually, always considered dizygotic, but we do not have the certainty of it [13]. DNA analysis, the gold standard for the confirmation of zygosity, is almost never required due to the high cost.
Dizygotic twins have been observed in ART after the transfer of a single embryo [14]. This can be explained by a single embryo transfer with a concurrent natural conception, which can occur both in fresh IVF cycles and in FET cycles without pituitary suppression. In our case, the women underwent down-regulated endometrial preparation protocol and no evidence of US ovulation signs were recorded. Moreover this is the first case, to our knowledge of triplet trichorionic triamniotic pregnancy from a single blastocyst transfer. How a multiple pregnancy with separate chorions can originate from a single blastocyst remains, to date, unknown. A previous study reported the visualization by time-lapse cinematography of an atypical hatch pattern of a vitrified blastocyst that led to two separate and complete blastocysts [15].
The micromanipulation of the zona pellucida during intracytoplasmic sperm injection (ICSI), preimplantation genetic screening/diagnosis (PGS / PGD) has been considered as a responsible factor. The process remains, to date, still a hypothesis: the herniation and subsequent split of the inner cell mass has been proposed as a possible mechanism [16-19]
Thanks to the ART and then the time-lapse spread it is possible to directly observe in the laboratories the embryo's development phases. Interestingly, to our knowledge, no embryologist reported an embryo spontaneous separation before the blastocyst stage [20-22] differently from what reported with the blastocyst [23]. The resulting monozygotic DCDA twin gestations challenge the axiom that only monochorionic gestations derive from the blastocyst.
Subsequent theories questioned Corner's dogma: Lopez-Moratalla hypothesizes that in monozygotic twin pregnancy there is a long process of fertilization with mitosis of the fertilized egg before polarization by calcium ions [24]. On this basis, Herranz hypothesized that in all monozygotic twinning, with the first division of the fertilized egg, twin zygotes are produced instead of blastomeres [3]. Therefore the placentation is explained not as a consequence of a division process but, on the contrary, as anomalous fusion of the membranes in the zona pellucida (monochorionic), or of the embryonic bodies (Siamese twins). If this fusion does not take place, both zygotes continue in the implantation as two independent blastocysts that will give birth to DCDA twins. Therefore monochorionic pregnancy could be the result of the fusion of the trophectoderm [25]. Scientific research on the proposed mechanism behind the monozygotic DCDA twinning must be continued. Therefore, this popular belief of chorionicity based uniquely on the day of embryonic development must be questioned.
Conclusions:
ART has drastically amplified the number of twin pregnancies which are, per se, pregnancies at risk of adverse neonatal and maternal outcomes. To overcome this issue, the practice of a single blastocyst transfer has become increasingly popular. However, some mechanisms on twinning are still unknown. Further researches on the etiology of monozygotic cleavage, as well as on the effect of in vitro fertilization techniques on embryonic division are mandatory. The blind acceptance of Corner's theory of embryonic division based on a merely temporal criterion must be updated on the basis of new acquisitions obtained with ART experiences. A careful study of the mechanisms underlying the late division of the blastocyst is necessary to ultimately reduce the risk of ART multiple monozygotic pregnancies.
Declarations
Funding: none
Conflicts of interest/Competing interests: none
Availability of data and material (data transparency): we declare transparency of data
Code availability (software application or custom code): N/A
Authors' contributions: All authors contributed to the study conception and design. Material preparationwas performed by Laura Badolato, data collection and analysis were performed by Elisabetta Micelli and Rossella Fucci and Elisabetta Coccia. The first draft of the manuscript was written by Elisabetta Micelli and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.