Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Clarós P1*, Mbonimpaye R2, Clarós A1, Pujol C1, Clarós-Pujol A1.
1Clínica Clarós, C/Los Vergós 31, 08017 Barcelona, Spain.
2University of Burundi. Intern in Otorhinolaryngology. Scholarship of Clarós Foundation
*Corresponding author: Pedro Clarós, Clínica Clarós, C/Los Vergós 31, 08017 Barcelona, Spain.
Received: July 13, 2021
Accepted: July 17, 2021
Published: July 22, 2021
Citation: Clarós P, Mbonimpaye R, Clarós A, Pujol C, Clarós-Pujol A “ Our first experience with a Cochlear Osia®OSI200 implant in Spain.” Clinical Case Reports and Clinical Study, 4(4); DOI: 10.61148/2766-8614/JCCRCS/088
Copyright: © 2021 Clarós P. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Various devices for hearing rehabilitation after bilateral conductive deafness due to ear malformation are available on the market. Cochlear companies developed new types of implantable devices with more efficacy and comfort for patients. Cochlear Osia® implant is a recent device with a new technology which offers both acoustic and aesthetic advantages. In this paper, we share our first experience with this new device in Spain in a 44-year-old patient. He suffered from a bilateral conductive hearing loss due to a bilateral middle and outer ear congenital malformation. Details on surgical procedure are given as well as outcomes in the patient.
INTRODUCTION
One of the methods used to address hearing loss, regardless of its origin, is bone conduction pathway. It may be indicated when outer and middle ear pathology does not allow for the use of a conventional hearing aid to improve hearing loss. A wide range of devices which transmit sound via bone pathway are available on the market to date (1).
We report the first case of new Cochlear Osia® implant in Spain in a patient with bilateral hearing loss due to external and middle ear congenital malformation.
CASE PRESENTATION
A Klippel Feil syndrome patient, 44 years old, with head, neck and ear malformation was received at our clinic, complaining about deep hearing loss. His twin brother is not affected by the Klippel Feil syndrome, but is affected by a bilateral conductive hearing loss for middle ear malformation too (Fig.1).

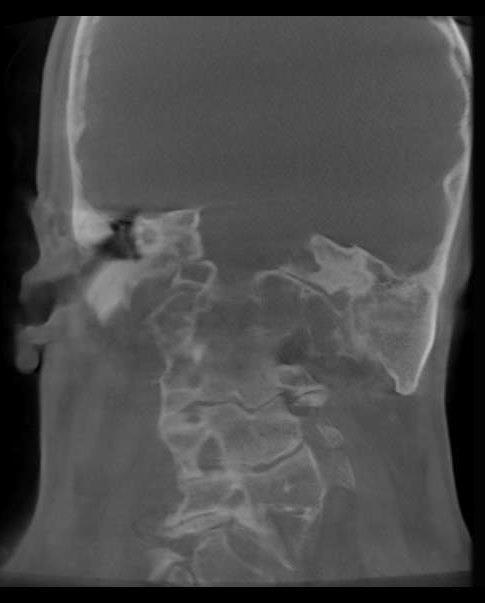
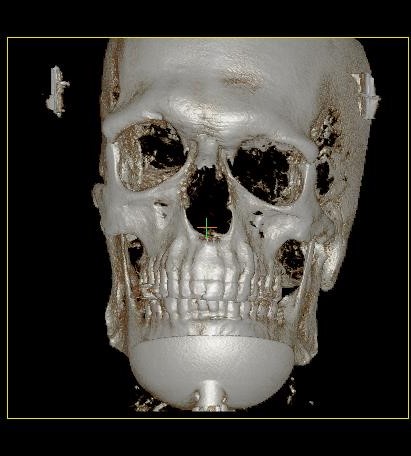
Figure 1. Major atresia left side, radiology and 3D Ct-Scan results.
The patient’s examination resulted in a skinny ear canal with a deep stenosis in the right ear as well as a minor aplasia of the pinna and major atresia of the ear canal in the left ear. The results of temporal bone CT-scan highlighted an asymmetry of the skull base with, on the left side, a narrow mastoid, poor pneumatization and atresia of external ear canal and middle ear. A better pneumatization of the right mastoid process was noted.
A tonal and vocal audiometry test helped us assess the depth of our patient’s hearing loss. A conductive hearing loss was noted in both ears, severe in the left (70 dBs) and mild in the right (45 dBs loss) (Fig.2).
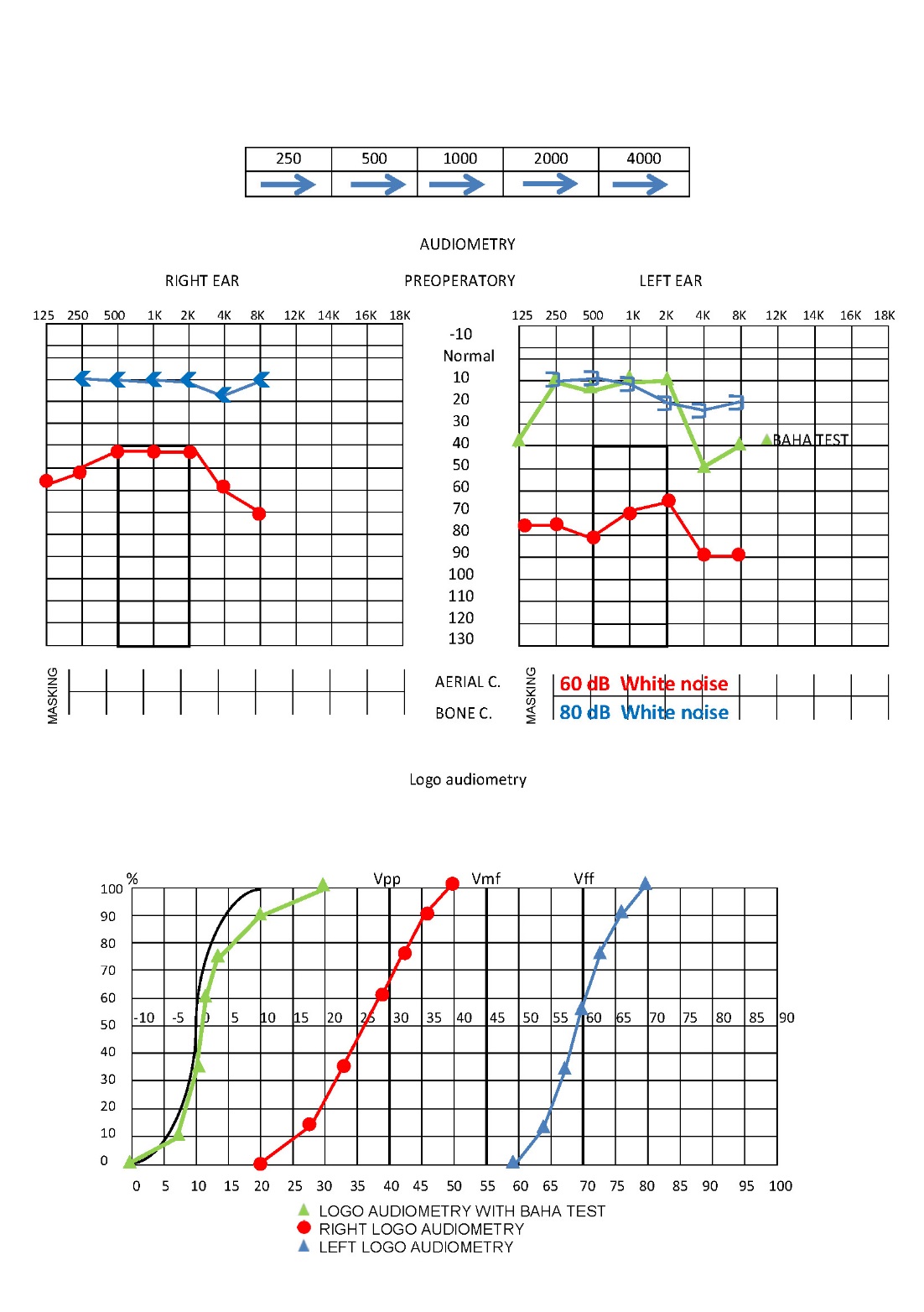
Figure 2. Tonal and vocal audiometry. Test of Baha.
The surgical kit includes OSI200 Implant, OSI200 Implant template, BI300 Implant, drills and non- magnetic plugs and replacement magnets.
The Osia® System consists of an external and internal (implanted) component (2):
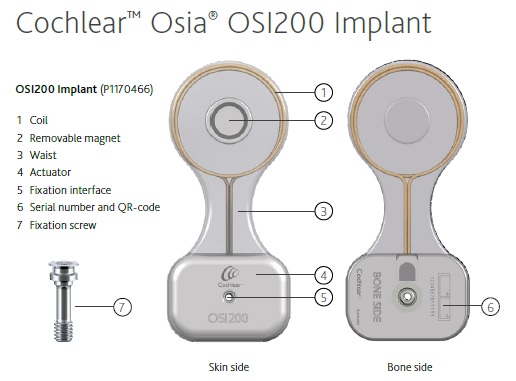
Figure 3: the bone conductive Cochlear Osia® Implant (2)
The surgical procedure follows five steps: preparation of the implant site, incision, coil pocket creation, BI300 Implant placement and OSI200 Implant placement, and closure.
The OSI200 Implant position is best when the actuator is placed close to and in horizontal line with the ear canal or slightly above without touching the pinna. The sound processor should not interfere with the pinna and the placement of glasses or be overlapped or shadowed by the pinna. We used the OSI200 Implant template to plan the correct position and marked the incision line on the skin. The hole of the actuator area of the OSI200 Implant template and a hypodermic needle with marking ink were used to mark the location of the BI300. To measure the soft tissue thickness we used a thin hypodermic needle, a clamp and a ruler before local anesthesia injection. We did inferior post-auricular incision with extension.
An incision down and through the periosteum was made to create the coil pocket and offer a tighter fit of the periosteum over the implant. We cleared away the periosteum around the BI300 Implant location using a small cruciate incision. During the drilling procedures, we used a drill indicator and abundant irrigation. We drilled at an angle perpendicular to the bone surface with up and down movement to ensure that irrigation reaches the tip of the drill. We applied a coolant to facilitate the osseointegration process. With the drill indicator in place, we inserted the implant at an angle perpendicular to the bone surface, without coolant, until the first threads of the implant were well within the bone (two rotations). Once in the bone, we continued placement with irrigation until the Osscora surgical set stopped automatically and beeped.
While maintaining the actuator with our fingers, we placed the center of the actuator on top of the BI300 Implant and hand-tightened the fixation screw with the screwdriver, avoiding the contact of the actuator with the bone. The skin and soft tissue were sutured in two separate layers and a pressure dressing was applied for 24 hours.
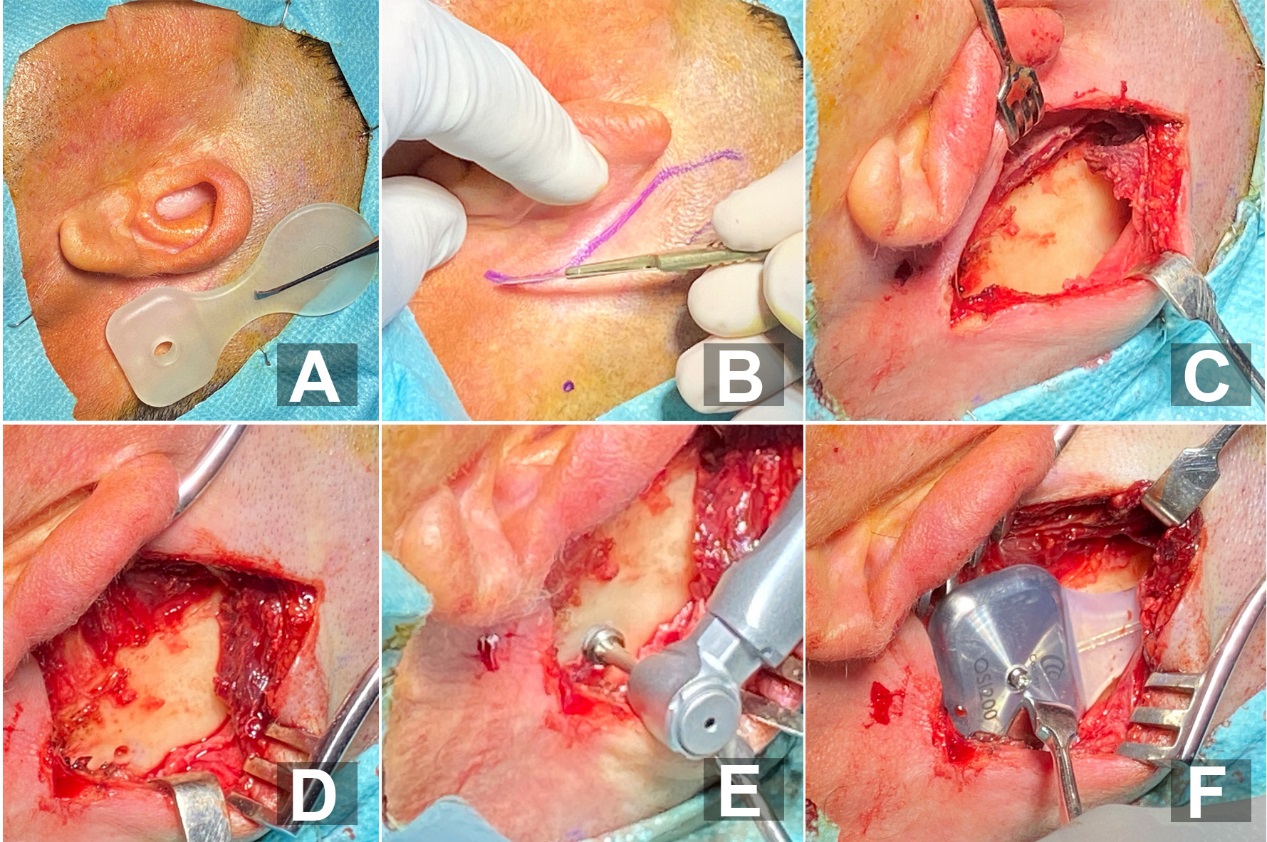
Figure 4. Different steps of surgery procedure: A) using the template to plan Osia® Implant position. B) Skin Incision C) Pocket Creation D) Exposure of the mastoid bone E) Drilling of the site of the BI300 in the mastoid bone F) Fixation of the implant on the bone .
The surgical procedure was successful without any postoperative complication. One week after surgery, the wound was clean and we removed suture. One month after surgery, the sound processor was programmed following the indications of the Cochlear Company by the Speech processor programmer of our Centre. We performed new Audiological tests which showed evident improvement. The patient was satisfied with both acoustic outcomes and aesthetic advantages of Cochlear Osia®OSI200 implant.
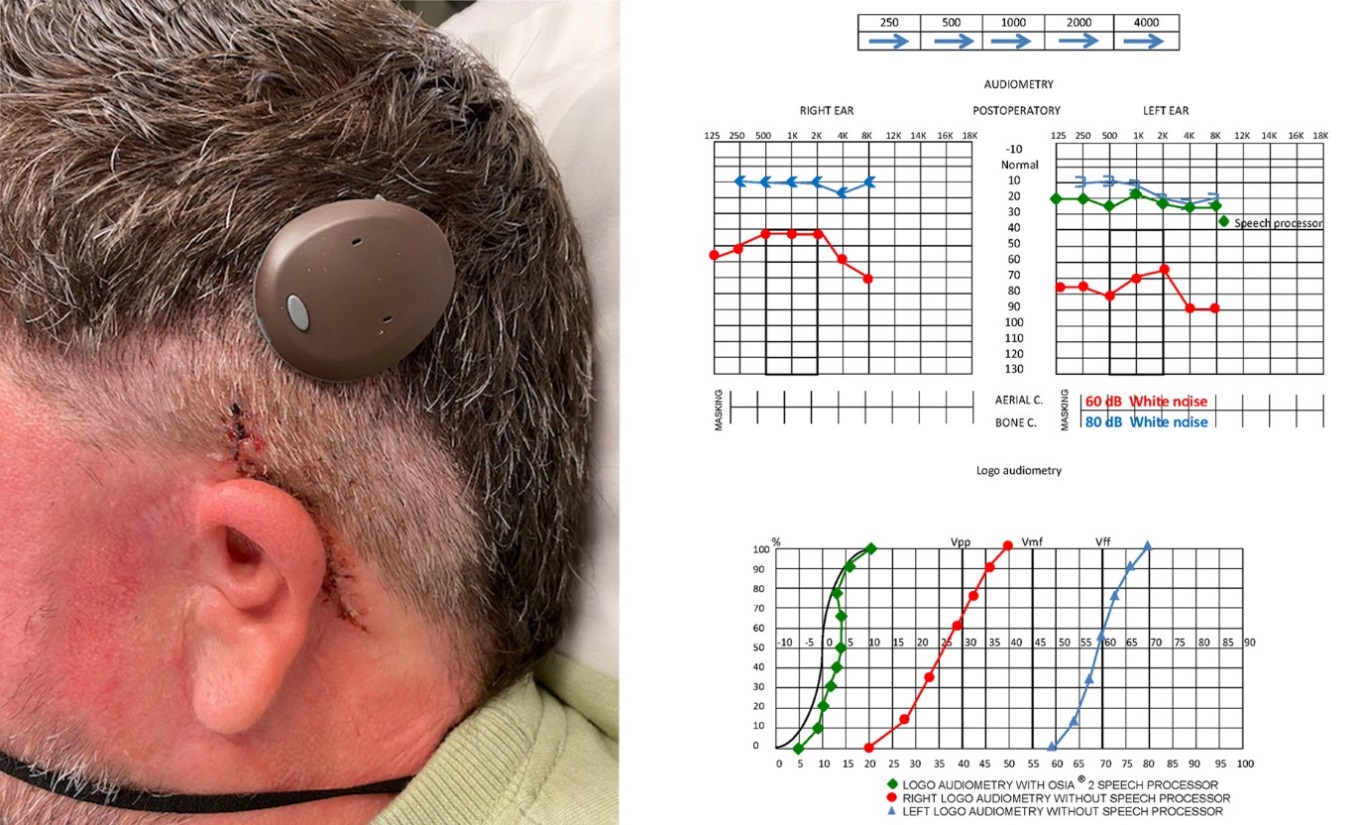
Figure 5. One month after surgery: patient and Audiological improvement
DISCUSSION
Two groups of conventional external hearing aids are available on the market: air-conduction and bone-conduction hearing aids. Air-conduction hearing aids require the use of suitable ear molds, which may be uncomfortable in patients with chronic middle ear and ear canal infections, atresia of the external canal, or an ear canal that cannot accommodate an ear mold. In the above cases, bone-conduction hearing aids should be a good option. In several studies, better hearing outcomes have been reported with bone-anchored hearing aids compared to external bone-conduction hearing devices (3).
According to the National Institute for Health and Care Excellence’s (NICE) guidelines, unilateral cochlear implantation is an option for patients with severe to profound deafness who do not receive adequate or cannot be helped with acoustic hearing aids (4). Due to bilateral external and middle ear malformation in our patient, acoustic aid had no interest. In a recent review study, significant improvement in quality of life was highlighted in implanted adults (5).
The bone-anchored hearing aid lets the patient improve hearing without occlusion or irritation of the ear canal. In particular, it is indicated for patients with congenital malformation of the outer and/or middle ear. This device offers the patients the possibility to have enhanced hearing in the presence of noisy background and to locate sound (6). Bilateral deafness in adults is one of several indications of Cochlear Osia® implant (2). We made it the first option in our patient who had a bilateral hearing loss due to bilateral outer and middle ear malformation.
In a study, comparison between Cochlear Osia® system and BAHA Attract® system has been done regarding improvement in the global profile of hearing aid benefit questionnaire and in the speech, spatial and qualities of hearing scale. The global benefits were more evident than in the Osia® group (in global score 49% vs. 37.2%)(7). Osia® implant, as well as BAHA Attract ® and Bonebridge®,has no infection hazard and presents aesthetic advantages because it is placed under the skin(8).
After cochlear Osia® implantation, possible complications are: general risks associated with surgery and general anesthesia, osseointegration failure, trauma, infection, generalized diseases and surgical complications, concurrent cerebrospinal fluid leakage, subdural injury, subcutaneous hematoma, irritation, inflammation or breakdown of the skin flap, infection and in some cases, extrusion of the device caused by the presence of a foreign body under the skin. Failure of device component parts (both external and internal) could result in the perception of an uncomfortable loud sound sensation, intermittent sound, or no sound by the patient. Removal or replacement of the implant can be contributive in these cases (2).
Early and late complications with cochlear implants have been reported in a recent study.
Surgical wound swelling, tinnitus or vertigo was the most frequent early complications. Infection was the most frequent complication later after implantation(9). In our patient, neither early, nor late postoperative complications have been registered. In our patient, hearing improvement was evident without aesthetic alteration.
CONCLUSION
Addressing conductive hearing loss has many options available on the market to date. However, depending on the patient’s case, the choice can be limited to the use of bone conduction pathway. Bone-anchored hearing aids are the best option, especially when outer and middle ear malformation does not allow for the use of classical hearing aids. Cochlear Osia® implant is made with a technology offering both functional and aesthetic advantages. In our patient, we made it our first choice and the outcomes were satisfying. Nevertheless, similar devices such as Bonebridge®, BAHA Attract® and PONTO® remain of great interest.
Author contributions
Clarós P, Mbonimpaye R, Clarós A, wrote the paper. Clarós P was the surgeon.
Pujol C and Clarós-Pujol A were respectively audiologist and speech programmer.
ORCIDs
Clarós P. 0000-0002-7567-0370
Clarós A. 0000-0001-6084-3470
Mbonimpaye R. 0000-0001-8792-3147
Conflicts of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could result in any potential conflict of interest.